We serve Chemical Name:2,3-Dichloro-1,1,1,3-tetrafluoropropane CAS:146916-90-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
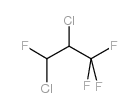
Chemical Name:2,3-Dichloro-1,1,1,3-tetrafluoropropane
CAS.NO:146916-90-7
Synonyms:2,3-dichloro-1,1,1,3-tetrafluoropropane
Molecular Formula:C3H2Cl2F4
Molecular Weight:184.94800
HS Code:
Physical and Chemical Properties:
Melting point:-98ºC
Boiling point:70ºC
Density:1.498g/cm3
Index of Refraction:1.344
PSA:
Exact Mass:183.94700
LogP:2.69050
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2,3-dichloro-1,1,1,3-tetrafluoropropane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,3-dichloro-1,1,1,3-tetrafluoropropane physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,3-dichloro-1,1,1,3-tetrafluoropropane Use and application,2,3-dichloro-1,1,1,3-tetrafluoropropane technical grade,usp/ep/jp grade.
Related News: You will find the name and amount of the active ingredient contained in the medicine on the package of OTC (over-the-counter) drugs. 2,3-Dichloro-1,1,1,3-tetrafluoropropane manufacturer The Company��s immuno-regulatory product candidates include ProTmune, a pharmacologically modulated, donor cell graft that is currently being evaluated in a Phase 2 clinical trial for the prevention of graft-versus-host disease, and a myeloid-derived suppressor cell immunotherapy for promoting immune tolerance in patients with immune disorders. Fate Therapeutics is headquartered in San Diego, CA. 2,3-Dichloro-1,1,1,3-tetrafluoropropane supplier You will find the name and amount of the active ingredient contained in the medicine on the package of OTC (over-the-counter) drugs. 2,3-Dichloro-1,1,1,3-tetrafluoropropane vendor Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country. 2,3-Dichloro-1,1,1,3-tetrafluoropropane factory The Company��s immuno-regulatory product candidates include ProTmune, a pharmacologically modulated, donor cell graft that is currently being evaluated in a Phase 2 clinical trial for the prevention of graft-versus-host disease, and a myeloid-derived suppressor cell immunotherapy for promoting immune tolerance in patients with immune disorders. Fate Therapeutics is headquartered in San Diego, CA.

