We serve Chemical Name:SPK-601 CAS:1096687-52-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
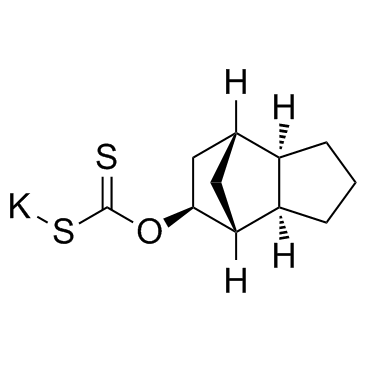
Chemical Name:SPK-601
CAS.NO:1096687-52-3
Synonyms:cs-0333;spk601
Molecular Formula:C11H15KOS2
Molecular Weight:266.46500
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:41.32000
Exact Mass:266.02000
LogP:2.65950
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like cs-0333 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,spk601 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,spk601 Use and application,spk601 technical grade,usp/ep/jp grade.
Related News: Compared with existing Imbruvica therapies, where patients take the BTK inhibitor daily—potentially for years—until their disease progresses, the new Venclexta combo caps treatment at about 14 months. That could offer a new option for “probably younger patients with CLL that prefer more flexible, treatment-free intervals,” said Craig Tendler, M.D., vice president of oncology clinical development and global medical affairs at J&J’s Janssen unit, during an interview. SPK-601 manufacturer Active pharmaceutical ingredients directly impact disease. SPK-601 supplier Australia’s ban followed an earlier move by the US Friday to deny entry to foreign nationals who have traveled in China in the last 14 days. SPK-601 vendor This also includes its anti-tau pipeline, with the FDA’s approval having “implications for AD programmes targeting Tau,” according to Jefferies. SPK-601 factory Compared with existing Imbruvica therapies, where patients take the BTK inhibitor daily—potentially for years—until their disease progresses, the new Venclexta combo caps treatment at about 14 months. That could offer a new option for “probably younger patients with CLL that prefer more flexible, treatment-free intervals,” said Craig Tendler, M.D., vice president of oncology clinical development and global medical affairs at J&J’s Janssen unit, during an interview.

