We serve Chemical Name:6-chloro-7-methylflavone CAS:147919-60-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
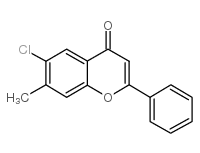
Chemical Name:6-chloro-7-methylflavone
CAS.NO:147919-60-6
Synonyms:MFCD00209563;6-chloro-7-methyl-2-phenylchromen-4-one;6-Chloro-7-methylflavone
Molecular Formula:C16H11ClO2
Molecular Weight:270.71000
HS Code:2932999099
Physical and Chemical Properties:
Melting point:178-180ºC(lit.)
Boiling point:435.3ºC at 760mmHg
Density:1.304g/cm3
Index of Refraction:1.631
PSA:30.21000
Exact Mass:270.04500
LogP:4.42180
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like MFCD00209563 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,6-Chloro-7-methylflavone physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,6-chloro-7-methyl-2-phenylchromen-4-one Use and application,6-Chloro-7-methylflavone technical grade,usp/ep/jp grade.
Related News: Fate Therapeutics, Inc. recently announced new in vivo preclinical data for FT819, its first off-the-shelf, iPSC-derived chimeric antigen receptor (CAR) T-cell product candidate, at the 61st American Society of Hematology (ASH) Meeting and Exposition in Orlando, FL. 6-chloro-7-methylflavone manufacturer Fate Therapeutics, Inc. recently announced new in vivo preclinical data for FT819, its first off-the-shelf, iPSC-derived chimeric antigen receptor (CAR) T-cell product candidate, at the 61st American Society of Hematology (ASH) Meeting and Exposition in Orlando, FL. 6-chloro-7-methylflavone supplier In the context of the new medicine policy, with the development of China’s R & D strength, the number of innovative drugs approved has been blown out, which further strengthened the demand and speed of industrialization of raw materials in China. 6-chloro-7-methylflavone vendor Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037. 6-chloro-7-methylflavone factory Without disclosing or confirming the number of vaccine doses, the FDA said in a news release that it had authorized two batches of the vaccine for use, that several other batches were not suitable for use and that others were being evaluated.

