We serve Chemical Name:Creatine ethyl ester hydrochloride CAS:15366-32-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
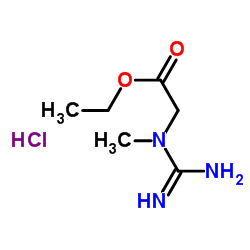
Chemical Name:Creatine ethyl ester hydrochloride
CAS.NO:15366-32-2
Synonyms:Ethyl N-carbamimidoyl-N-methylglycinate hydrochloride (1:1);Glycine, N-(aminoiminomethyl)-N-methyl-, ethyl ester, hydrochloride (1:1)
Molecular Formula:C6H14ClN3O2
Molecular Weight:195.647
HS Code:2925290090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:79.41000
Exact Mass:195.077454
LogP:0.97680
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Ethyl N-carbamimidoyl-N-methylglycinate hydrochloride (1:1) chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Glycine, N-(aminoiminomethyl)-N-methyl-, ethyl ester, hydrochloride (1:1) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Ethyl N-carbamimidoyl-N-methylglycinate hydrochloride (1:1) Use and application,Ethyl N-carbamimidoyl-N-methylglycinate hydrochloride (1:1) technical grade,usp/ep/jp grade.
Related News: The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market. Creatine ethyl ester hydrochloride manufacturer What is the difference? Raw material refers to chemical compounds that are used as a base to make an API. Creatine ethyl ester hydrochloride supplier Same as Imbruvica’s original monotherapy use, the Venclexta combo offers a convenient oral treatment, while the drug’s two other approved cocktails—with Gazyva or Roche’s Rituxan—involve infusions. Creatine ethyl ester hydrochloride vendor The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market. Creatine ethyl ester hydrochloride factory This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician��s Choice plus Best Supportive Care.

