We serve Chemical Name:2,3-Diethylpyrazine CAS:15707-24-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
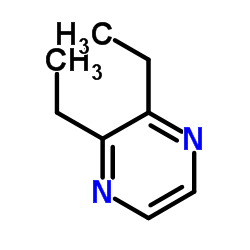
Chemical Name:2,3-Diethylpyrazine
CAS.NO:15707-24-1
Synonyms:2,3-Diethylpyrazine;tetramethyl pyrazine;MFCD00006151;EINECS 239-800-1;2,3-Diethyl-pyrazine;FEMA No. 3136;2,3-Diethyl-pyrazin;Pyrazine, 2,3-diethyl-;2,3,6-TRIMETHOXY-BENZALDEHYDE;Pyrazine,2,3-diethyl
Molecular Formula:C8H12N2
Molecular Weight:136.194
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:183.8±35.0 °C at 760 mmHg
Density:1.0±0.1 g/cm3
Index of Refraction:1.499
PSA:25.78000
Exact Mass:136.100052
LogP:1.70
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 3334
Packing Group:
Contact us for information like 2,3-Diethylpyrazine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Pyrazine,2,3-diethyl physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,3-Diethylpyrazine Use and application,FEMA No. 3136 technical grade,usp/ep/jp grade.
Related News: Dr. Anthony Fauci, director of the U.S. National Institute of Allergy and Infectious Diseases, said, It’s really very impressive,” noting that the vaccine was as good as the most effective shots developed so far during the pandemic. “It’s very important for the world’s population to have, yet again, another highly efficacious vaccine that looks in its trial to have a good safety profile,” Fauci told the Washington Post.
As heartening as the results were, the vaccine may not become a key player in the pandemic until late summer or fall.
Erck told the Post that Novavax will apply for regulatory clearance from a half-dozen countries in the third quarter, which begins in July. With tens of millions of doses already in hand, the company plans to boost manufacturing to produce 100 million doses a month by the end of September and 150 million doses a month in the last three months of the year.
In the United States, the company still needs to file for emergency authorization. The data, which was presented in a news release, will be examined by regulators at the U.S. Food and Drug Administration and by an advisory committee of vaccine advisers. Erck said the vaccine will likely have its biggest initial impact globally, through the World Health Organization’s COVAX initiative.
“A lot of our vaccine is going to be targeted in the early stages for COVAX … and so a lot of those doses are going to get into the low- and middle-income countries first, which is a good thing,” Erck said. Novavax has pledged 1.1 billion doses to COVAX.
The Novavax vaccine was one of six candidates the U.S. government made a huge bet on, investing $1.6 billion to pay for research and development and preordering 110 million doses, the Post reported.
In January, a large U.K. trial showed it was nearly 90% effective, even once a more transmissible variant had taken hold. Over the past five months, health officials and scientists have waited anxiously for confirming evidence from the U.S. trial. But that second study did not start until the end of December, due in part to manufacturing delays.
Meanwhile, the United States had secured more than enough shots from the three companies with authorized vaccines — Pfizer, Moderna and Johnson & Johnson — to satisfy demand. A fourth, from AstraZeneca, reported results in March.
Recombinant protein vaccines such as Novavax’s — the hepatitis B vaccine is another example — teach the immune system to recognize a virus by introducing a lab-made version of a viral protein.
Once the production process is in place, the vaccine offers potential advantages.
“The benefit of their formulation … is it’s remarkably scalable, so they can scale to a very high number of doses,” Matthew Frieman, a coronavirus expert at the University of Maryland’s School of Medicine who has worked with the company in the past, told the Post. “It’s not a super-strange production platform … you don’t need super-specialized facilities. It’s stable, so you don’t need a severe cold chain” to store the vaccine, he said.
The American South and Midwest are home to the highest obesity rates in the nation, but a new study reveals that severely obese residents of those regions are the least likely to choose lifesaving weight-loss surgery.
“Bariatric surgery has been shown to provide long-term weight loss, sustained improvements in cardiovascular and metabolic health, and even prolonged longevity,” noted study author Dr. Scott Schimpke, but the analysis “shows we continue to underutilize the best treatment for morbid obesity and associated metabolic syndrome. 2,3-Diethylpyrazine manufacturer Also, the study did not compare people who had COVID-19 with those who did not, to see if such symptoms were higher than in the general population. The report did exclude patients with certain serious or chronic preexisting conditions like cancer, kidney disease, HIV, liver disease and stroke, to separate their previous health status from post-COVID symptoms. 2,3-Diethylpyrazine supplier Britons being brought from Wuhan to the UK are being placed in quarantine at Arrowe Park Hospital in the Wirral, northwest England, Raab said. 2,3-Diethylpyrazine vendor “The synergies among our Regulatory, Clinical and Real-World Evidence (RWE) practices and CROS NT’s global biometrics capabilities will enhance Alira Health’s unique ability to serve clients across their solutions lifecycle,” said CEO Gabriele Brambilla in a statement. 2,3-Diethylpyrazine factory In a memo to Branchburg employees on May 20, Nellie Clark, head of the plant, summed up the outside lawyers’ findings: “In the end and very importantly, allegations that Lilly made false statements to the FDA were not substantiated.”
As heartening as the results were, the vaccine may not become a key player in the pandemic until late summer or fall.
Erck told the Post that Novavax will apply for regulatory clearance from a half-dozen countries in the third quarter, which begins in July. With tens of millions of doses already in hand, the company plans to boost manufacturing to produce 100 million doses a month by the end of September and 150 million doses a month in the last three months of the year.
In the United States, the company still needs to file for emergency authorization. The data, which was presented in a news release, will be examined by regulators at the U.S. Food and Drug Administration and by an advisory committee of vaccine advisers. Erck said the vaccine will likely have its biggest initial impact globally, through the World Health Organization’s COVAX initiative.
“A lot of our vaccine is going to be targeted in the early stages for COVAX … and so a lot of those doses are going to get into the low- and middle-income countries first, which is a good thing,” Erck said. Novavax has pledged 1.1 billion doses to COVAX.
The Novavax vaccine was one of six candidates the U.S. government made a huge bet on, investing $1.6 billion to pay for research and development and preordering 110 million doses, the Post reported.
In January, a large U.K. trial showed it was nearly 90% effective, even once a more transmissible variant had taken hold. Over the past five months, health officials and scientists have waited anxiously for confirming evidence from the U.S. trial. But that second study did not start until the end of December, due in part to manufacturing delays.
Meanwhile, the United States had secured more than enough shots from the three companies with authorized vaccines — Pfizer, Moderna and Johnson & Johnson — to satisfy demand. A fourth, from AstraZeneca, reported results in March.
Recombinant protein vaccines such as Novavax’s — the hepatitis B vaccine is another example — teach the immune system to recognize a virus by introducing a lab-made version of a viral protein.
Once the production process is in place, the vaccine offers potential advantages.
“The benefit of their formulation … is it’s remarkably scalable, so they can scale to a very high number of doses,” Matthew Frieman, a coronavirus expert at the University of Maryland’s School of Medicine who has worked with the company in the past, told the Post. “It’s not a super-strange production platform … you don’t need super-specialized facilities. It’s stable, so you don’t need a severe cold chain” to store the vaccine, he said.
The American South and Midwest are home to the highest obesity rates in the nation, but a new study reveals that severely obese residents of those regions are the least likely to choose lifesaving weight-loss surgery.
“Bariatric surgery has been shown to provide long-term weight loss, sustained improvements in cardiovascular and metabolic health, and even prolonged longevity,” noted study author Dr. Scott Schimpke, but the analysis “shows we continue to underutilize the best treatment for morbid obesity and associated metabolic syndrome. 2,3-Diethylpyrazine manufacturer Also, the study did not compare people who had COVID-19 with those who did not, to see if such symptoms were higher than in the general population. The report did exclude patients with certain serious or chronic preexisting conditions like cancer, kidney disease, HIV, liver disease and stroke, to separate their previous health status from post-COVID symptoms. 2,3-Diethylpyrazine supplier Britons being brought from Wuhan to the UK are being placed in quarantine at Arrowe Park Hospital in the Wirral, northwest England, Raab said. 2,3-Diethylpyrazine vendor “The synergies among our Regulatory, Clinical and Real-World Evidence (RWE) practices and CROS NT’s global biometrics capabilities will enhance Alira Health’s unique ability to serve clients across their solutions lifecycle,” said CEO Gabriele Brambilla in a statement. 2,3-Diethylpyrazine factory In a memo to Branchburg employees on May 20, Nellie Clark, head of the plant, summed up the outside lawyers’ findings: “In the end and very importantly, allegations that Lilly made false statements to the FDA were not substantiated.”

