We serve Chemical Name:5,5′-methylenebis(1H-benzotriazole) CAS:15805-10-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
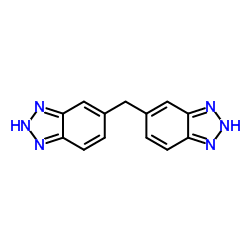
Chemical Name:5,5′-methylenebis(1H-benzotriazole)
CAS.NO:15805-10-4
Synonyms:5,5′-methylene-bis(benzotriazole);1H-1,2,3-benzotriazole, 6,6′-methylenebis-;5,5′-methylenebis(1H-benzotriazole);Methane,bis(5-benzotriazolyl);1H-Benzotriazole,5,5′-methylenebis;Bis-(1H-benzotriazol-5-yl)-methan;2H-1,2,3-Benzotriazole, 5,5′-methylenebis-;1H-1,2,3-benzotriazole, 5,5′-methylenebis-;5,5′-Methylenebis(2H-benzotriazole);6,6′-Methylenebis(1H-benzotriazole);bis-(1H-benzotriazol-5-yl)-methane;5,5′-methylenebis-1H-Benzotriazole
Molecular Formula:C13H10N6
Molecular Weight:250.259
HS Code:2933990090
Physical and Chemical Properties:
Melting point:238-240 ºC
Boiling point:543.1±60.0 °C at 760 mmHg
Density:1.502
Index of Refraction:1.819
PSA:83.14000
Exact Mass:250.096695
LogP:2.06
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 5,5′-methylene-bis(benzotriazole) chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,5,5′-methylenebis-1H-Benzotriazole physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,bis-(1H-benzotriazol-5-yl)-methane Use and application,5,5′-methylene-bis(benzotriazole) technical grade,usp/ep/jp grade.
Related News: The second is known as the excipient, which is the inactive substance that serves as the vehicle for the API itself. 5,5′-methylenebis(1H-benzotriazole) manufacturer The second is known as the excipient, which is the inactive substance that serves as the vehicle for the API itself. 5,5′-methylenebis(1H-benzotriazole) supplier The second is known as the excipient, which is the inactive substance that serves as the vehicle for the API itself. 5,5′-methylenebis(1H-benzotriazole) vendor Analysts at Jefferies, meanwhile, said the FDA accelerated approval for Aduhelm, which was based on a surrogate endpoint of amyloid beta plaque reduction (and not clinical benefit) “has implications for ongoing AD studies,” most notably, it reckons, for Roche’s phase 3 GRADUATE test for its anti-amyloid-beta candidate gantenerumab, “as a much lower hurdle than demonstrating clear cognitive benefit” has now become precedent. Jefferies, still cautious, said it remains “unclear what stance FDA may take for the field if GRADUATE fails on cognition despite significant Abeta reductions,” but says it’s probably not going to revive Roche-AC Immune’s anti-amyloid-beta candidate crenezumab, which saw its late-stage CREAD trials discontinued for futility but which is currently in an Alzheimer’s prevention study. 5,5′-methylenebis(1H-benzotriazole) factory Analysts at Jefferies, meanwhile, said the FDA accelerated approval for Aduhelm, which was based on a surrogate endpoint of amyloid beta plaque reduction (and not clinical benefit) “has implications for ongoing AD studies,” most notably, it reckons, for Roche’s phase 3 GRADUATE test for its anti-amyloid-beta candidate gantenerumab, “as a much lower hurdle than demonstrating clear cognitive benefit” has now become precedent. Jefferies, still cautious, said it remains “unclear what stance FDA may take for the field if GRADUATE fails on cognition despite significant Abeta reductions,” but says it’s probably not going to revive Roche-AC Immune’s anti-amyloid-beta candidate crenezumab, which saw its late-stage CREAD trials discontinued for futility but which is currently in an Alzheimer’s prevention study.

