We serve Chemical Name:2-Amino-3,4-difluorobenzoic acid CAS:158580-94-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
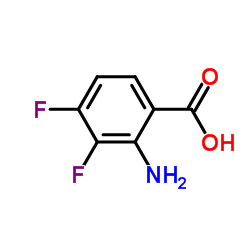
Chemical Name:2-Amino-3,4-difluorobenzoic acid
CAS.NO:158580-94-0
Synonyms:Benzoic acid, 2-amino-3,4-difluoro-;2-Amino-3,4-difluorobenzoic acid
Molecular Formula:C7H5F2NO2
Molecular Weight:173.117
HS Code:2922499990
Physical and Chemical Properties:
Melting point:N/A
Boiling point:303.0±42.0 °C at 760 mmHg
Density:1.5±0.1 g/cm3
Index of Refraction:1.578
PSA:63.32000
Exact Mass:173.028839
LogP:2.25
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Benzoic acid, 2-amino-3,4-difluoro- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Amino-3,4-difluorobenzoic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Amino-3,4-difluorobenzoic acid Use and application,2-Amino-3,4-difluorobenzoic acid technical grade,usp/ep/jp grade.
Related News: This will entail workforce hiring of over 110 personnel too. Benzoyloxy-cyanessigsaeure-aethylester manufacturers Zynteglo was granted conditional marketing authorisation in May 2019. The conditional authorisation means that there is more evidence to come about the medicine, which the company will provide. EMA regularly reviews new information that becomes available. 8-Bromo-6-propyl-3H-imidazo[1,5-d][1,2,4]triazin-4-one suppliers Specifically, the EC has cleared Verquvo (vericiguat) to treat patients who are stabilised after a recent decompensation event requiring intravenous therapy. 4H-Imidazo[4,5,1-ij]quinoline, 5,6-dihydro-2-[(2-pyridinylmethyl)thio]- vendor & factory This will entail workforce hiring of over 110 personnel too.,Zynteglo was granted conditional marketing authorisation in May 2019. The conditional authorisation means that there is more evidence to come about the medicine, which the company will provide. EMA regularly reviews new information that becomes available.

