We serve Chemical Name:Montelukast CAS:158966-92-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
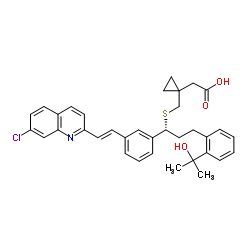
Chemical Name:Montelukast
CAS.NO:158966-92-8
Synonyms:Cyclopropaneacetic acid, 1-[[[(1R)-1-[3-[(E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]-;[3H]-Montelukast;Brondilat (TN);Montelukast;(1-{1-{3(R)-[2-(7-chloro-quinolin-2-yl)-vinyl]-phenyl}-3-[2-(1-hydroxy-1-methyl-ethyl)-phenyl]-propylsulfanylmethyl}-cyclopropyl)-acetic acid;{1-[({(1R)-1-{3-[(E)-2-(7-Chloroquinolin-2-yl)vinyl]phenyl}-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetic acid;Montair;2-[1-[[(1R)-1-[3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl]sulfanylmethyl]cyclopropyl]acetic acid;Singular;[R-(E)]-1-[[[1-[3-[2-(7-Chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic Acid;{1-[({(1R)-1-{3-[(E)-2-(7-Chloro-2-quinolinyl)vinyl]phenyl}-3-[2-(2-hydroxy-2-propanyl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetic acid;Montelukast [INN:BAN];MFCD05662278;Montelukast (INN);{1-[({(1R)-1-{3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl}-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetic acid;[14C]-Montelukast;UNII-MHM278SD3E
Molecular Formula:C35H36ClNO3S
Molecular Weight:586.183
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:750.5±60.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.678
PSA:95.72000
Exact Mass:585.210449
LogP:7.80
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Cyclopropaneacetic acid, 1-[[[(1R)-1-[3-[(E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,UNII-MHM278SD3E physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,[R-(E)]-1-[[[1-[3-[2-(7-Chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic Acid Use and application,[R-(E)]-1-[[[1-[3-[2-(7-Chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic Acid technical grade,usp/ep/jp grade.
Related News: Lilly did show much more conclusively that donanemab, which zeroes in on amyloid plaques, does reduce levels of the aggregates of misfolded proteins in the brain. This is what Biogen also showed with Aduhelm, and it turned out this week to be enough for a full FDA approval, hence the stock bump for Lilly. Montelukast manufacturer Lilly did show much more conclusively that donanemab, which zeroes in on amyloid plaques, does reduce levels of the aggregates of misfolded proteins in the brain. This is what Biogen also showed with Aduhelm, and it turned out this week to be enough for a full FDA approval, hence the stock bump for Lilly. Montelukast supplier China’s basic chemical industry is developed, and the intermediate industry has also entered a mature stage. It is basically able to independently support the production needs of various domestic drug substances, which also brings cost advantages to Chinese drug substance companies. On the other hand, if there is insufficient supply of upstream products or price fluctuations, the sales of API products will also fluctuate in volume and price. Montelukast vendor Lilly did show much more conclusively that donanemab, which zeroes in on amyloid plaques, does reduce levels of the aggregates of misfolded proteins in the brain. This is what Biogen also showed with Aduhelm, and it turned out this week to be enough for a full FDA approval, hence the stock bump for Lilly. Montelukast factory From the perspective of the overall life cycle of generic drugs from R & D to sales, with the expiration of patents, the business model for the production and marketing of generic pharmaceutical raw materials has changed, demand has increased, business potential has been realized quickly, and gross profit margins have shown a downward trend.

