We serve Chemical Name:MAGE-3 (271-279) CAS:160295-81-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
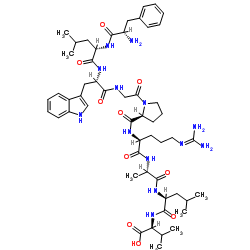
Chemical Name:MAGE-3 (271-279)
CAS.NO:160295-81-8
Synonyms:L-Valine, L-phenylalanyl-L-leucyl-L-tryptophylglycyl-L-prolyl-N-(diaminomethylene)-L-ornithyl-L-alanyl-L-leucyl-;L-Phenylalanyl-L-leucyl-L-tryptophylglycyl-L-prolyl-N-(diaminomethylene)-L-ornithyl-L-alanyl-L-leucyl-L-valine
Molecular Formula:C53H79N13O10
Molecular Weight:1058.276
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:1.4±0.1 g/cm3
Index of Refraction:1.642
PSA:365.02000
Exact Mass:1057.607300
LogP:4.48
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like L-Valine, L-phenylalanyl-L-leucyl-L-tryptophylglycyl-L-prolyl-N-(diaminomethylene)-L-ornithyl-L-alanyl-L-leucyl- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,L-Phenylalanyl-L-leucyl-L-tryptophylglycyl-L-prolyl-N-(diaminomethylene)-L-ornithyl-L-alanyl-L-leucyl-L-valine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,L-Phenylalanyl-L-leucyl-L-tryptophylglycyl-L-prolyl-N-(diaminomethylene)-L-ornithyl-L-alanyl-L-leucyl-L-valine Use and application,L-Phenylalanyl-L-leucyl-L-tryptophylglycyl-L-prolyl-N-(diaminomethylene)-L-ornithyl-L-alanyl-L-leucyl-L-valine technical grade,usp/ep/jp grade.
Related News: But even though weight-loss success depends on adopting a challenging” change in post-operation diet and lifestyle, the process can certainly “help people to lose weight and get comorbid [other negative health] conditions under better control,” Sandon said.
So it’s important, she said, to help eligible patients better “understand the treatment options and potential for success,” by sharing surgery success stories and highlighting the benefits of reducing high-risk health issues such as blood pressure and diabetes.
An experimental Alzheimer’s vaccine appears to safely clear abnormal tau protein from the brain, but it’s not yet clear whether the shot will be able to save brain function.
In a Phase 2 clinical trial, the vaccine produced high levels of antibodies to target and attack free-floating tau proteins before they can form “tau tangles” that clog neurons and damage brain function. Tau tangles, along with plaques formed by the protein amyloid-beta, serve as one of the main hallmarks of Alzheimer’s.
“While amyloid influences speed of Alzheimer’s progression, there is strong evidence that tau pathology relates to the underlying cause of the disease,” said lead researcher Dr. Petr Novak, a senior clinical research scientist at AXON Neuroscience, the Slovakian pharmaceutical company developing the vaccine. “Brain atrophy and cognitive loss closely echo the deposition of pathological tau protein, as evidenced by recent tau PET studies. MAGE-3 (271-279) manufacturer Pharmaceutical intermediates: chemical raw materials or chemical products used in the process of pharmaceutical synthesis, are intermediate products in the process of producing APIs, and can be further processed into APIs. MAGE-3 (271-279) supplier The downstream industry of the pharmaceutical intermediate industry is mainly the production of APIs, and the relationship between APIs and preparations is in the upstream and downstream industry chain. The consumer demand for downstream preparations will directly affect the demand for APIs. MAGE-3 (271-279) vendor But they’ll be subject to up to 14 days of mandatory quarantine once they’re back in the US. MAGE-3 (271-279) factory But even though weight-loss success depends on adopting a challenging” change in post-operation diet and lifestyle, the process can certainly “help people to lose weight and get comorbid [other negative health] conditions under better control,” Sandon said.
So it’s important, she said, to help eligible patients better “understand the treatment options and potential for success,” by sharing surgery success stories and highlighting the benefits of reducing high-risk health issues such as blood pressure and diabetes.
An experimental Alzheimer’s vaccine appears to safely clear abnormal tau protein from the brain, but it’s not yet clear whether the shot will be able to save brain function.
In a Phase 2 clinical trial, the vaccine produced high levels of antibodies to target and attack free-floating tau proteins before they can form “tau tangles” that clog neurons and damage brain function. Tau tangles, along with plaques formed by the protein amyloid-beta, serve as one of the main hallmarks of Alzheimer’s.
“While amyloid influences speed of Alzheimer’s progression, there is strong evidence that tau pathology relates to the underlying cause of the disease,” said lead researcher Dr. Petr Novak, a senior clinical research scientist at AXON Neuroscience, the Slovakian pharmaceutical company developing the vaccine. “Brain atrophy and cognitive loss closely echo the deposition of pathological tau protein, as evidenced by recent tau PET studies.
So it’s important, she said, to help eligible patients better “understand the treatment options and potential for success,” by sharing surgery success stories and highlighting the benefits of reducing high-risk health issues such as blood pressure and diabetes.
An experimental Alzheimer’s vaccine appears to safely clear abnormal tau protein from the brain, but it’s not yet clear whether the shot will be able to save brain function.
In a Phase 2 clinical trial, the vaccine produced high levels of antibodies to target and attack free-floating tau proteins before they can form “tau tangles” that clog neurons and damage brain function. Tau tangles, along with plaques formed by the protein amyloid-beta, serve as one of the main hallmarks of Alzheimer’s.
“While amyloid influences speed of Alzheimer’s progression, there is strong evidence that tau pathology relates to the underlying cause of the disease,” said lead researcher Dr. Petr Novak, a senior clinical research scientist at AXON Neuroscience, the Slovakian pharmaceutical company developing the vaccine. “Brain atrophy and cognitive loss closely echo the deposition of pathological tau protein, as evidenced by recent tau PET studies. MAGE-3 (271-279) manufacturer Pharmaceutical intermediates: chemical raw materials or chemical products used in the process of pharmaceutical synthesis, are intermediate products in the process of producing APIs, and can be further processed into APIs. MAGE-3 (271-279) supplier The downstream industry of the pharmaceutical intermediate industry is mainly the production of APIs, and the relationship between APIs and preparations is in the upstream and downstream industry chain. The consumer demand for downstream preparations will directly affect the demand for APIs. MAGE-3 (271-279) vendor But they’ll be subject to up to 14 days of mandatory quarantine once they’re back in the US. MAGE-3 (271-279) factory But even though weight-loss success depends on adopting a challenging” change in post-operation diet and lifestyle, the process can certainly “help people to lose weight and get comorbid [other negative health] conditions under better control,” Sandon said.
So it’s important, she said, to help eligible patients better “understand the treatment options and potential for success,” by sharing surgery success stories and highlighting the benefits of reducing high-risk health issues such as blood pressure and diabetes.
An experimental Alzheimer’s vaccine appears to safely clear abnormal tau protein from the brain, but it’s not yet clear whether the shot will be able to save brain function.
In a Phase 2 clinical trial, the vaccine produced high levels of antibodies to target and attack free-floating tau proteins before they can form “tau tangles” that clog neurons and damage brain function. Tau tangles, along with plaques formed by the protein amyloid-beta, serve as one of the main hallmarks of Alzheimer’s.
“While amyloid influences speed of Alzheimer’s progression, there is strong evidence that tau pathology relates to the underlying cause of the disease,” said lead researcher Dr. Petr Novak, a senior clinical research scientist at AXON Neuroscience, the Slovakian pharmaceutical company developing the vaccine. “Brain atrophy and cognitive loss closely echo the deposition of pathological tau protein, as evidenced by recent tau PET studies.

