We serve Chemical Name:FGF-401 CAS:1708971-55-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
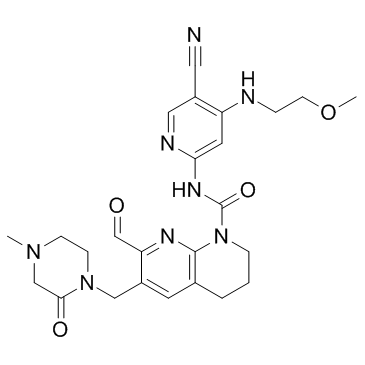
Chemical Name:FGF-401
CAS.NO:1708971-55-4
Synonyms:1,8-Naphthyridine-1(2H)-carboxamide, N-[5-cyano-4-[(2-methoxyethyl)amino]-2-pyridinyl]-7-formyl-3,4-dihydro-6-[(4-methyl-2-oxo-1-piperazinyl)methyl]-;N-{5-Cyano-4-[(2-methoxyethyl)amino]-2-pyridinyl}-7-formyl-6-[(4-methyl-2-oxo-1-piperazinyl)methyl]-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxamide;FGF401
Molecular Formula:C25H30N8O4
Molecular Weight:506.557
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:852.1±65.0 °C at 760 mmHg
Density:1.4±0.1 g/cm3
Index of Refraction:1.654
PSA:
Exact Mass:506.239014
LogP:-0.15
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1,8-Naphthyridine-1(2H)-carboxamide, N-[5-cyano-4-[(2-methoxyethyl)amino]-2-pyridinyl]-7-formyl-3,4-dihydro-6-[(4-methyl-2-oxo-1-piperazinyl)methyl]- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,FGF401 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,N-{5-Cyano-4-[(2-methoxyethyl)amino]-2-pyridinyl}-7-formyl-6-[(4-methyl-2-oxo-1-piperazinyl)methyl]-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxamide Use and application,FGF401 technical grade,usp/ep/jp grade.
Related News: Active Pharmaceutical Ingredients (APIs): Pharmaceutical active ingredients, which are the basic substances that constitute the pharmacological effects of pharmaceuticals, and are prepared by chemical synthesis, plant extraction, or biotechnology. FGF-401 manufacturer Active Pharmaceutical Ingredients (APIs): Pharmaceutical active ingredients, which are the basic substances that constitute the pharmacological effects of pharmaceuticals, and are prepared by chemical synthesis, plant extraction, or biotechnology. FGF-401 supplier It is understood that at present, the industry competition for global characteristic APIs has shown a development trend of vertical integration, and the number of mergers and acquisitions between pharmaceutical manufacturers and API manufacturers is increasing. FGF-401 vendor ��Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations�� (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials. FGF-401 factory An FDA inspection also turned up a long list of sanitary problems and bad manufacturing practices at the Emergent plant.

