We serve Chemical Name:Varespladib CAS:172732-68-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
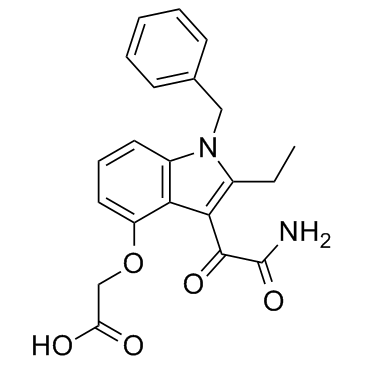
Chemical Name:Varespladib
CAS.NO:172732-68-2
Synonyms:Acetic acid, 2-[[3-(2-amino-1,2-dioxoethyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]-;({3-[Amino(oxo)acetyl]-1-benzyl-2-ethyl-1H-indol-4-yl}oxy)acetic acid;Varespladib;N-(3,4-dimethoxyphenyl)-6,7-dimethoxyquinazolin-4-amine
Molecular Formula:C21H20N2O5
Molecular Weight:380.394
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:667.9±65.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.630
PSA:111.62000
Exact Mass:380.137207
LogP:2.45
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like Acetic acid, 2-[[3-(2-amino-1,2-dioxoethyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,N-(3,4-dimethoxyphenyl)-6,7-dimethoxyquinazolin-4-amine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,N-(3,4-dimethoxyphenyl)-6,7-dimethoxyquinazolin-4-amine Use and application,N-(3,4-dimethoxyphenyl)-6,7-dimethoxyquinazolin-4-amine technical grade,usp/ep/jp grade.
Related News: Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country. Varespladib manufacturer What is the difference? Raw material refers to chemical compounds that are used as a base to make an API. Varespladib supplier Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country. Varespladib vendor Active pharmaceutical ingredients or APIs can be defined as the chemicals used to manufacture pharmaceutical drugs. Varespladib factory Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

