We serve Chemical Name:N~1~-(2,4-dichlorophenyl)ethanediamide CAS:17738-96-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
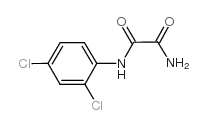
Chemical Name:N~1~-(2,4-dichlorophenyl)ethanediamide
CAS.NO:17738-96-4
Synonyms:N’-(2,4-dichlorophenyl)oxamide
Molecular Formula:C8H6Cl2N2O2
Molecular Weight:233.05100
HS Code:2924299090
Physical and Chemical Properties:
Melting point:233-235ºC
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:72.19000
Exact Mass:231.98100
LogP:2.19050
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like N’-(2,4-dichlorophenyl)oxamide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,N’-(2,4-dichlorophenyl)oxamide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,N’-(2,4-dichlorophenyl)oxamide Use and application,N’-(2,4-dichlorophenyl)oxamide technical grade,usp/ep/jp grade.
Related News: He said the FDA made its decision by fiat, and had not asked its advisors to consider whether the drug’s ability to remove a type of brain plaques known as beta amyloid would improve outcomes for patients. N~1~-(2,4-dichlorophenyl)ethanediamide manufacturer It has reserves in the direction of APIs and intermediates for lowering blood lipids, lowering blood sugar and anticoagulation. N~1~-(2,4-dichlorophenyl)ethanediamide supplier Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance. N~1~-(2,4-dichlorophenyl)ethanediamide vendor Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country. N~1~-(2,4-dichlorophenyl)ethanediamide factory Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

