We serve Chemical Name:Sulfacytine CAS:17784-12-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
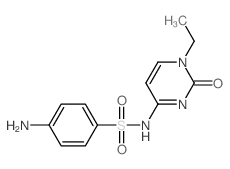
Chemical Name:Sulfacytine
CAS.NO:17784-12-2
Synonyms:Sulfacitinum [INN-Latin];4-amino-N-(1-ethyl-2-oxo-1,2-dihydro-pyrimidin-4-yl)-benzenesulfonamide;Sulfacitinum;1-Ethyl N4-sulfanilylcytosin;4-amino-N-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)benzenesulfonamide;Sulfacytine;CI-636;Renoquid;N-Sulfanilyl-1-ethylcytosin;Sulfacitina [INN-Spanish];Sulfacitine;Sulfacitina;N-sulfanilyl-1-ethylcytosine;1-Ethyl-N-sulfanilylcytosine
Molecular Formula:C12H14N4O3S
Molecular Weight:294.33000
HS Code:2935009090
Physical and Chemical Properties:
Melting point:166.5-168ºC
Boiling point:496.8ºC at 760mmHg
Density:1.45g/cm3
Index of Refraction:1.664
PSA:115.46000
Exact Mass:294.07900
LogP:2.38120
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Sulfacitinum [INN-Latin] chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1-Ethyl-N-sulfanilylcytosine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-amino-N-(1-ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)benzenesulfonamide Use and application,Renoquid technical grade,usp/ep/jp grade.
Related News: After a four-year battle over a cancer drug patent, Novartis has been ordered by a California judge to pay a Daiichi Sankyo subsidiary $177.8 million. sulfasalazine manufacturers In the US, however, ODAC in April voted to keep alive the accelerated approval for Tecentriq plus Abraxane (nab-paclitaxel) in mTNBC while additional confirmatory trials are ongoing. Trityl Candesartan Cilexetil suppliers In response, the strategy sets out how government will work with institutions, businesses, funders and charities to drive change in the sector, with new initiatives to encourage more young people into research, broaden career pathways in the sector and look further into the impacts of bureaucracy on researchers. Flusulfamide vendor & factory “The Agency also noted that no effect was seen in the two studies that included patients from EU populations, including the most recent study which involved patients who were receiving the maximum and optimal treatment for their Parkinson’s disease,” EMA said.,The Ballina facility was first established in 1974 and was acquired by Charles River in 2002. Over the years it has added to its capabilities offering a comprehensive package of GMP services in support of recombinant biologics, vaccines, cell and gene therapies, biosimilars, and medical devices. Charles River now employs 230 people at two facilities in Ireland: the site in Ballina Co. Mayo which focuses on biologics testing, and a site in Dublin, established in 2017, which serves as the EMEA and APAC headquarters for the Company’s Microbial Solutions division.

