We serve Chemical Name:Bis(benzyloxy)(dichloro)silane CAS:18414-52-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
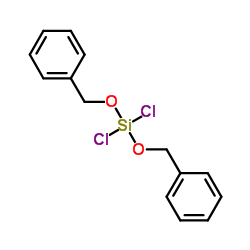
Chemical Name:Bis(benzyloxy)(dichloro)silane
CAS.NO:18414-52-3
Synonyms:Dibenzyloxy-dichlorsilan;Silane,dichlorobis(phenylmethoxy);DIBENZYLOXYDICHLOROSILANE;Benzene, 1,1′-[(dichlorosilylene)bis(oxymethylene)]bis-;Silane,bis(benzyloxy)dichloro-(6CI,8CI);Silane,dichlorobis(phenylmethoxy)-(9CI);Bis(benzyloxy)(dichloro)silane
Molecular Formula:C14H14Cl2O2Si
Molecular Weight:313.251
HS Code:2931900090
Physical and Chemical Properties:
Melting point:>0ºC
Boiling point:360.7±31.0 °C at 760 mmHg
Density:1.2±0.1 g/cm3
Index of Refraction:1.560
PSA:18.46000
Exact Mass:312.014008
LogP:7.64
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2987
Packing Group:
Contact us for information like Dibenzyloxy-dichlorsilan chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Bis(benzyloxy)(dichloro)silane physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Bis(benzyloxy)(dichloro)silane Use and application,Silane,dichlorobis(phenylmethoxy)-(9CI) technical grade,usp/ep/jp grade.
Related News: The U.S. laws governing cosmetics safety and labelling date back to 1938 and 1967, which tells you something,” Bruton said.
However, on Tuesday, Sens. Susan Collins (R-Maine) and Richard Blumenthal (D-Conn.) introduced the No PFAS in Cosmetics Act in the Senate, the Washington Post reported. The same bill was introduced in the U.S. House by Rep. Debbie Dingell (D-Michigan). It would direct the U.S. Food and Drug Administration to issue a proposed rule banning the intentional addition of PFAS in cosmetics within 270 days of the bill’s passage, and require a final rule to be issued 90 days later.
“Our bill would require the FDA to ban the addition of PFAS to cosmetics products,” Collins said in a statement. “Americans should be able to trust that the products they are applying to their hair or skin are safe. Bis(benzyloxy)(dichloro)silane manufacturer In recent years, with the increasing number of patent medicines whose patents have expired, the variety and quantity of generic drugs have also increased rapidly, which has brought huge market opportunities to the API market and the output of APIs has continued to increase. Bis(benzyloxy)(dichloro)silane supplier AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment. Bis(benzyloxy)(dichloro)silane vendor AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment. Bis(benzyloxy)(dichloro)silane factory Government prosecutors often consider whether a corporation made a genuine effort to investigate and address potentially illegal activities once learning of them.
However, on Tuesday, Sens. Susan Collins (R-Maine) and Richard Blumenthal (D-Conn.) introduced the No PFAS in Cosmetics Act in the Senate, the Washington Post reported. The same bill was introduced in the U.S. House by Rep. Debbie Dingell (D-Michigan). It would direct the U.S. Food and Drug Administration to issue a proposed rule banning the intentional addition of PFAS in cosmetics within 270 days of the bill’s passage, and require a final rule to be issued 90 days later.
“Our bill would require the FDA to ban the addition of PFAS to cosmetics products,” Collins said in a statement. “Americans should be able to trust that the products they are applying to their hair or skin are safe. Bis(benzyloxy)(dichloro)silane manufacturer In recent years, with the increasing number of patent medicines whose patents have expired, the variety and quantity of generic drugs have also increased rapidly, which has brought huge market opportunities to the API market and the output of APIs has continued to increase. Bis(benzyloxy)(dichloro)silane supplier AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment. Bis(benzyloxy)(dichloro)silane vendor AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment. Bis(benzyloxy)(dichloro)silane factory Government prosecutors often consider whether a corporation made a genuine effort to investigate and address potentially illegal activities once learning of them.

