We serve Chemical Name:tert-butyl 4-ethyl-3-oxopiperazine-1-carboxylate CAS:194350-95-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
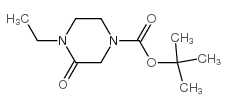
Chemical Name:tert-butyl 4-ethyl-3-oxopiperazine-1-carboxylate
CAS.NO:194350-95-3
Synonyms:1-ETHYL-4-(TERT-BUTYLOXYCARBONYL)PIPERAZIN-2-ONE;4-ethyl-3-oxopiperazine-1-carboxylic acid tert-butyl ester;1-tert-butoxycarbonyl-4-ethyl-3-oxopiperazine
Molecular Formula:C11H20N2O3
Molecular Weight:228.28800
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:348ºC at 760 mmHg
Density:1.087 g/cm3
Index of Refraction:
PSA:49.85000
Exact Mass:228.14700
LogP:0.96140
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1-ETHYL-4-(TERT-BUTYLOXYCARBONYL)PIPERAZIN-2-ONE chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1-tert-butoxycarbonyl-4-ethyl-3-oxopiperazine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1-tert-butoxycarbonyl-4-ethyl-3-oxopiperazine Use and application,1-ETHYL-4-(TERT-BUTYLOXYCARBONYL)PIPERAZIN-2-ONE technical grade,usp/ep/jp grade.
Related News: The Japanese drugmaker is injecting $126 million into its Thousand Oaks facility to boost manufacturing and support new product lines, Pacific Coast Business Times first reported. Takeda was joined by the city’s mayor, Claudia Bill-de la Peña, for a groundbreaking event on Thursday, July 22. 4-Bromo-2-(bromomethyl)pyridine manufacturers Japan on Friday approved the administration of AstraZeneca Plc’s (AZN.L) COVID-19 vaccine after a few months’ pause due to fears about potential side effects, local media reported. DL-α-BROMO-β-(5-IMIDAZOLYL)PROPIONIC ACID suppliers In the trial, 362 eligible patients were randomised (1:1) and received a fixed-dose intravenous infusion of 300mg anifrolumab or placebo every four weeks. 1-[2-[(3-cyano-8-methylquinolin-2-yl)amino]ethyl]-1-(2-methylphenyl)urea vendor & factory.

