We serve Chemical Name:Fmoc-D-tert-leucine CAS:198543-64-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
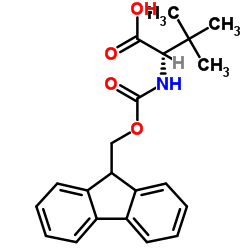
Chemical Name:Fmoc-D-tert-leucine
CAS.NO:198543-64-5
Synonyms:(2R)-2-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}-3,3-dimethylbutanoic acid;Fmoc-L-tert-leucine;Fmoc-D-tert-Leu-OH;Fmoc-(D-tert-leucine);Fmoc-tBu-Gly-OH;Fmoc-D-Tle-OH;L-Valine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-3-methyl-;N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-3-methyl-L-valine;Fmoc-tert-leucine;(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3,3-dimethylbutanoic acid
Molecular Formula:C21H23NO4
Molecular Weight:353.412
HS Code:
Physical and Chemical Properties:
Melting point:126-130ºC
Boiling point:554.1±33.0 °C at 760 mmHg
Density:1.2±0.1 g/cm3
Index of Refraction:1.584
PSA:75.63000
Exact Mass:353.162720
LogP:4.77
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (2R)-2-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}-3,3-dimethylbutanoic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3,3-dimethylbutanoic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-3-methyl-L-valine Use and application,L-Valine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-3-methyl- technical grade,usp/ep/jp grade.
Related News: EMA said the drug can help improve the respiratory function of Pompe disease patients, and the most common side effects include hypersensitivity (including anaphylaxis) and infusion-associated reactions. 2-{4-[5-Benzyloxy-2-(2,4-dimethoxy-phenyl)-3-methyl-indol-1-ylmethyl]-phenoxy}-ethanol manufacturers Conducted by Innovent in China, the CIBI321A101 trial is a Phase 1a open-label, multi-center study of the safety, tolerability and primary efficacy of IBI321 in patients with advanced solid tumors. Phase 1a of the study will evaluate dosing of IBI321 in a variety of solid tumors (ClinicalTrials.gov, NCT04911894). trans-dichloro-(2,6-dimethylpyridine)(dimethylsulfide)-platinum (II) suppliers The outlay will be used to grow Takeda’s portfolio of treatments and boost capacity to manufacture additional products for the rare disease community, Stephen Hatke, Takeda’s Thousand Oaks site head, said in a YouTube video about the expansion. The company didn’t name the specific products it plans to make there. 2-(RS)-(2-aminobenzoylamino)succinic acid diethyl ester vendor & factory.

