We serve Chemical Name:Fmoc-D-Phe(F5)-OH CAS:198545-85-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
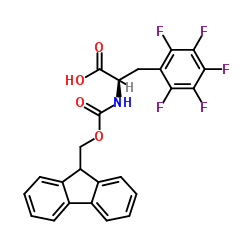
Chemical Name:Fmoc-D-Phe(F5)-OH
CAS.NO:198545-85-6
Synonyms:AmbotzFAA1686;Fmoc-L-phe(F5)-OH;MFCD00672558;Fmoc-2,3,4,5,6-pentafluoro-D-phenylalanine;FMOC-D-PENTAFLUOROPHENYLALANINE;(2R)-2-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}-3-(pentafluorophenyl)propanoic acid;D-Phenylalanine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-2,3,4,5,6-pentafluoro-;N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-2,3,4,5,6-pentafluoro-D-phenylalanine;N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-2,3,4,5,6-pentafluoro-L-phenylalanine;Fmoc-D-2,3,4,5,6-Pentafluorophenylalanine;Fmoc-L-2,3,4,5,6-Pentafluorophenylalanine;Fmoc-3-Pentafluorophenyl-D-alanine;D-Pentafluorophenylalanine,N-FMOC protected;L-Phenylalanine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-2,3,4,5,6-pentafluoro-;Fmoc-D-Phe(F5)-OH
Molecular Formula:C24H16F5NO4
Molecular Weight:477.380
HS Code:
Physical and Chemical Properties:
Melting point:156-158ºC
Boiling point:602.9±55.0 °C at 760 mmHg
Density:1.5±0.1 g/cm3
Index of Refraction:1.583
PSA:75.63000
Exact Mass:477.099945
LogP:5.59
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like AmbotzFAA1686 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Fmoc-D-Phe(F5)-OH physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Fmoc-L-2,3,4,5,6-Pentafluorophenylalanine Use and application,Fmoc-D-2,3,4,5,6-Pentafluorophenylalanine technical grade,usp/ep/jp grade.
Related News: This means that the drug attributes of the drug substance will be lost in the future, and the monopoly power of some drug substances will also be lost. The preparation company will become the main person in charge of the drug. The drug preparation company will be responsible for the quality of the original excipients. It will be more cautious, some raw and auxiliary materials companies whose quality cannot be guaranteed will be gradually eliminated, and the industry concentration will be further improved. Fmoc-D-Phe(F5)-OH manufacturer The two trials studied similar patient populations, Tendler said, but VMP was an old standard of care that’s not really used today in the U.S. Still, Tendler believes the two regimens may both have roles to play based on each patient’s ability to tolerate them. Whichever cocktail physicians choose, the two phase 3 studies have confirmed the benefit of Darzalex in transplant-ineligible patients, he added. Fmoc-D-Phe(F5)-OH supplier The program caused the price of some drugs to plunge more than 90% when it was introduced last year in some cities, state news agency Xinhua said. Fmoc-D-Phe(F5)-OH vendor It was not clear which inquiry – the company’s or the government’s – was launched first and to what extent the subjects coincide. However, as Reuters reported last month, based on three sources familiar with the matter, the Justice Department’s investigation into Lilly includes a review of alleged manufacturing irregularities and records-tampering. Fmoc-D-Phe(F5)-OH factory This means that the drug attributes of the drug substance will be lost in the future, and the monopoly power of some drug substances will also be lost. The preparation company will become the main person in charge of the drug. The drug preparation company will be responsible for the quality of the original excipients. It will be more cautious, some raw and auxiliary materials companies whose quality cannot be guaranteed will be gradually eliminated, and the industry concentration will be further improved.

