We serve Chemical Name:3-Phenoxybenzhydrazide CAS:206761-84-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
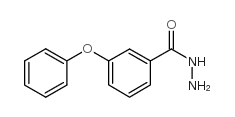
Chemical Name:3-Phenoxybenzhydrazide
CAS.NO:206761-84-4
Synonyms:3-PHENOXYBENZHYDRAZIDE;3-phenoxybenzohydrazide
Molecular Formula:C13H12N2O2
Molecular Weight:228.24700
HS Code:2928000090
Physical and Chemical Properties:
Melting point:108-110ºC
Boiling point:N/A
Density:1.217g/cm3
Index of Refraction:1.612
PSA:64.35000
Exact Mass:228.09000
LogP:3.17360
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 3-PHENOXYBENZHYDRAZIDE chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-phenoxybenzohydrazide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-phenoxybenzohydrazide Use and application,3-phenoxybenzohydrazide technical grade,usp/ep/jp grade.
Related News: Cases recorded in Thailand, Taiwan, Germany, Vietnam, Japan, France and the United States involved patients who had not been to China. 3-Phenoxybenzhydrazide manufacturer The April halt followed the discovery that ingredients from AstraZeneca’s (AZN.L) COVID-19 vaccine, also being produced at the plant at the time, contaminated a batch of J&J’s vaccine. AstraZeneca’s shot is no longer being made there. 3-Phenoxybenzhydrazide supplier The April halt followed the discovery that ingredients from AstraZeneca’s (AZN.L) COVID-19 vaccine, also being produced at the plant at the time, contaminated a batch of J&J’s vaccine. AstraZeneca’s shot is no longer being made there. 3-Phenoxybenzhydrazide vendor The risk-based score generated by the tool helps sponsors gain advance understanding of the appropriate level of clinical supply management oversight needed to help get their project started on time and maintain momentum as the study progresses. 3-Phenoxybenzhydrazide factory Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.

