We serve Chemical Name:Ethyl 5-cyclopropyl-1,2-oxazole-3-carboxylate CAS:21080-81-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
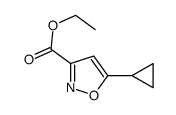
Chemical Name:Ethyl 5-cyclopropyl-1,2-oxazole-3-carboxylate
CAS.NO:21080-81-9
Synonyms:Ethyl 5-cyclopropylisoxazole-3-carboxylate
Molecular Formula:C9H11NO3
Molecular Weight:181.18900
HS Code:2934999090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:52.33000
Exact Mass:181.07400
LogP:1.72870
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Ethyl 5-cyclopropylisoxazole-3-carboxylate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Ethyl 5-cyclopropylisoxazole-3-carboxylate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Ethyl 5-cyclopropylisoxazole-3-carboxylate Use and application,Ethyl 5-cyclopropylisoxazole-3-carboxylate technical grade,usp/ep/jp grade.
Related News: One key question for the fixed-duration combo is whether it can sustain remission after treatment has stopped. In the preliminary analysis of the GLOW trial, at three months after treatment ended, 51.9% of the Imbruvica-Venclexta patients had undetectable measurable residual disease (uMRD)—a stringent marker reflecting no signs of cancer—in bone marrow, versus 17.1% for the control group. The rates for peripheral blood were 54.7% and 39% for the two groups, respectively. Ethyl 5-cyclopropyl-1,2-oxazole-3-carboxylate manufacturer One key question for the fixed-duration combo is whether it can sustain remission after treatment has stopped. In the preliminary analysis of the GLOW trial, at three months after treatment ended, 51.9% of the Imbruvica-Venclexta patients had undetectable measurable residual disease (uMRD)—a stringent marker reflecting no signs of cancer—in bone marrow, versus 17.1% for the control group. The rates for peripheral blood were 54.7% and 39% for the two groups, respectively. Ethyl 5-cyclopropyl-1,2-oxazole-3-carboxylate supplier Active pharmaceutical ingredients or APIs can be defined as the chemicals used to manufacture pharmaceutical drugs. Ethyl 5-cyclopropyl-1,2-oxazole-3-carboxylate vendor One key question for the fixed-duration combo is whether it can sustain remission after treatment has stopped. In the preliminary analysis of the GLOW trial, at three months after treatment ended, 51.9% of the Imbruvica-Venclexta patients had undetectable measurable residual disease (uMRD)—a stringent marker reflecting no signs of cancer—in bone marrow, versus 17.1% for the control group. The rates for peripheral blood were 54.7% and 39% for the two groups, respectively. Ethyl 5-cyclopropyl-1,2-oxazole-3-carboxylate factory he agency said the drugmaker and Emergent must agree that the FDA can share relevant information about the manufacturing of the doses with regulators where the vaccine is shipped.

