We serve Chemical Name:2-(4-Bromobutyl)oxirane CAS:21746-88-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
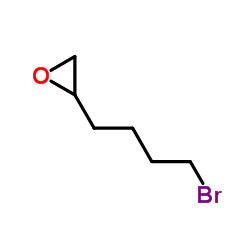
Chemical Name:2-(4-Bromobutyl)oxirane
CAS.NO:21746-88-3
Synonyms:2-(4-Bromobutyl)oxirane;6-Bromo-1,2-epoxy-hexane;6-Bromo-1,2-epoxyhexan;Oxirane, 2-(4-bromobutyl)-;6-Brom-1-hexenoxid
Molecular Formula:C6H11BrO
Molecular Weight:179.055
HS Code:2910900090
Physical and Chemical Properties:
Melting point:-22ºC
Boiling point:197.2±13.0 °C at 760 mmHg
Density:1.4±0.1 g/cm3
Index of Refraction:1.492
PSA:12.53000
Exact Mass:177.999313
LogP:1.79
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-(4-Bromobutyl)oxirane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,6-Brom-1-hexenoxid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,6-Brom-1-hexenoxid Use and application,6-Bromo-1,2-epoxy-hexane technical grade,usp/ep/jp grade.
Related News: The slowdown of pesticide intermediates has dragged down the growth of industrial business. In the future, the pharmaceutical intermediate business will be the focus of the company’s development. 2-(4-Bromobutyl)oxirane manufacturer The slowdown of pesticide intermediates has dragged down the growth of industrial business. In the future, the pharmaceutical intermediate business will be the focus of the company’s development. 2-(4-Bromobutyl)oxirane supplier The slowdown of pesticide intermediates has dragged down the growth of industrial business. In the future, the pharmaceutical intermediate business will be the focus of the company’s development. 2-(4-Bromobutyl)oxirane vendor At Day 35 following administration, a bone marrow assessment showed that FT819 persisted and continued to demonstrate tumor clearance, whereas primary CAR T cells, while persisting, were not able to control tumor growth. 2-(4-Bromobutyl)oxirane factory The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.

