We serve Chemical Name:H-Gly-Pro-Hyp-OH CAS:2239-67-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
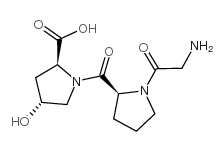
Chemical Name:H-Gly-Pro-Hyp-OH
CAS.NO:2239-67-0
Synonyms:trans-1-(1-Glycyl-L-prolyl)-4-hydroxy-L-prolin;Glycylprolylhydroxyproline;trans-1-(1-glycyl-L-prolyl)-4-hydroxy-L-proline;GLY-PRO-HYDROXY-PRO
Molecular Formula:C12H19N3O5
Molecular Weight:285.29600
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:629.8ºC at 760mmHg
Density:1.493g/cm3
Index of Refraction:1.619
PSA:124.17000
Exact Mass:285.13200
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like trans-1-(1-Glycyl-L-prolyl)-4-hydroxy-L-prolin chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,GLY-PRO-HYDROXY-PRO physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,trans-1-(1-glycyl-L-prolyl)-4-hydroxy-L-proline Use and application,trans-1-(1-glycyl-L-prolyl)-4-hydroxy-L-proline technical grade,usp/ep/jp grade.
Related News: After several years of R & D and improvement, the production process of commonly used generic drug bulk drugs is relatively mature, and the products of similar companies have high similarities. Therefore, the competitive advantage of bulk drug companies is mainly reflected in cost control. Companies with cost advantages can usually pass Competition to expand production capacity and further gain scale advantages, while having stable, high-quality upstream supply. H-Gly-Pro-Hyp-OH manufacturer The API can be directly formulated, and the intermediate can only be used to synthesize the next product. Only through the intermediate can the API be manufactured. H-Gly-Pro-Hyp-OH supplier The API can be directly formulated, and the intermediate can only be used to synthesize the next product. Only through the intermediate can the API be manufactured. H-Gly-Pro-Hyp-OH vendor The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy. H-Gly-Pro-Hyp-OH factory The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.

