We serve Chemical Name:pentafluoro-λ5-stibane,sulfurofluoridic acid CAS:23854-38-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
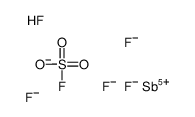
Chemical Name:pentafluoro-λ5-stibane,sulfurofluoridic acid
CAS.NO:23854-38-8
Synonyms:Fluorosulfuric acid-antimony pentafluoride 1:1;MFCD09039262;EINECS 245-915-8;Fluorosulfuric acid-antimony pentafluoride 4:1;Magic Acid
Molecular Formula:F6HO3SSb
Molecular Weight:316.82200
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:65.58000
Exact Mass:315.85900
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2922 8/PG 1
Packing Group:
Contact us for information like Fluorosulfuric acid-antimony pentafluoride 1:1 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Magic Acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Fluorosulfuric acid-antimony pentafluoride 1:1 Use and application,Fluorosulfuric acid-antimony pentafluoride 4:1 technical grade,usp/ep/jp grade.
Related News: The researchers found that compared with those with cancer, the prevalence of FT was higher among those with ASCVD (54 versus 41 percent). In adjusted analyses, when studying individual components of FT, those with ASCVD had increased odds of any difficulty paying medical bills, inability to pay bills, cost-related medication nonadherence, food insecurity, and foregone/delayed care due to cost (odds ratios, 1.22, 1.25, 1.28, 1.39, and 1.17, respectively). pentafluoro-λ5-stibane,sulfurofluoridic acid manufacturer For example, there are concerns that PFAS’ effect on the thyroid could lead to thyroid cancer, van Gerwen said. pentafluoro-λ5-stibane,sulfurofluoridic acid supplier With the global industrial division of labor and the change in the business model of multinational pharmaceutical companies, the outsourced market for patented drug substances will further expand. pentafluoro-λ5-stibane,sulfurofluoridic acid vendor In a statement to Fierce Medtech, Innova said it has completed some corrective actions, while others are still underway, and that the U.S. recall was launched to reclaim tests distributed to employees, clinical studies and to customers for early evaluation. The company said it plans to seek an emergency use authorization and comply with all FDA requirements. pentafluoro-λ5-stibane,sulfurofluoridic acid factory With the global industrial division of labor and the change in the business model of multinational pharmaceutical companies, the outsourced market for patented drug substances will further expand.

