We serve Chemical Name:ditert-butyl(propan-2-yl)phosphane CAS:25032-49-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
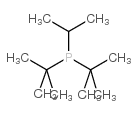
Chemical Name:ditert-butyl(propan-2-yl)phosphane
CAS.NO:25032-49-9
Synonyms:di-tert-butylisopropylphosphine;di-t-butylisopropylphosphine;Phosphine,di-tert-butylisopropyl
Molecular Formula:C11H25P
Molecular Weight:188.29000
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:13.59000
Exact Mass:188.16900
LogP:4.47370
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like di-tert-butylisopropylphosphine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Phosphine,di-tert-butylisopropyl physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,di-t-butylisopropylphosphine Use and application,Phosphine,di-tert-butylisopropyl technical grade,usp/ep/jp grade.
Related News: Green Valley said it would launch the drug ��very soon�� in China. The company also aims to roll out a phase-3 clinical trial with sites in the United States, Europe and Asia in early 2020 to facilitate global regulatory approval of the drug. ditert-butyl(propan-2-yl)phosphane manufacturer The Darzalex-Rd regimen got its FDA go-ahead in transplant-ineligible patients in 2019 based on data from the same phase 3 MAIA trial showing it could pare down the risk of disease progression or death by 44% after a median follow-up of 28 months. Now, after 56.2 months of follow-up, over half of Darzalex patients were still alive without their disease worsening, a showing that’s again “unprecedented” for a front-line myeloma treatment, Tendler said. ditert-butyl(propan-2-yl)phosphane supplier This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.? ditert-butyl(propan-2-yl)phosphane vendor The StartScore model was developed by the company��s in-house supply chain experts based on actual performance data. ditert-butyl(propan-2-yl)phosphane factory Green Valley said it would launch the drug ��very soon�� in China. The company also aims to roll out a phase-3 clinical trial with sites in the United States, Europe and Asia in early 2020 to facilitate global regulatory approval of the drug.

