We serve Chemical Name:3-pyrimidin-2-ylpropan-1-ol CAS:260441-09-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
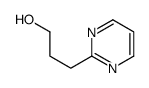
Chemical Name:3-pyrimidin-2-ylpropan-1-ol
CAS.NO:260441-09-6
Synonyms:2-Pyrimidinepropanol;3-(2-pyrimidyl)propan-1-ol;2-Pyrimidinepropanol (9CI);3-(2-pyrimidinyl)propanol;3-PYRIMIDIN-2-YL-PROPAN-1-OL
Molecular Formula:C7H10N2O
Molecular Weight:138.16700
HS Code:2933599090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:46.01000
Exact Mass:138.07900
LogP:0.40150
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-Pyrimidinepropanol chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-PYRIMIDIN-2-YL-PROPAN-1-OL physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Pyrimidinepropanol Use and application,3-(2-pyrimidyl)propan-1-ol technical grade,usp/ep/jp grade.
Related News: Fate Therapeutics�� iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications. 3-pyrimidin-2-ylpropan-1-ol manufacturer First of all, as an API manufacturer we think of how to make a chemical compound which becomes an API in the laboratory. 3-pyrimidin-2-ylpropan-1-ol supplier Travel alerts: As the Wuhan coronavirus spreads around China, infecting more people, a number of countries have raised their travel advisory warnings. 3-pyrimidin-2-ylpropan-1-ol vendor Fate Therapeutics�� iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications. 3-pyrimidin-2-ylpropan-1-ol factory INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.

