We serve Chemical Name:Palladium(2+) nitrite ammoniate (1:2:2) CAS:28068-05-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
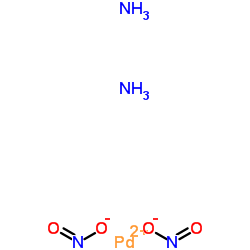
Chemical Name:Palladium(2+) nitrite ammoniate (1:2:2)
CAS.NO:28068-05-5
Synonyms:Aminodibenzanthrone;Palladium(2+) nitrite ammoniate (1:2:2);Violanthrone,amino;EINECS 247-989-7;Aminoviolanthrone
Molecular Formula:C34H17NO2
Molecular Weight:232.492
HS Code:
Physical and Chemical Properties:
Melting point:230ºC(lit.)
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:60.16000
Exact Mass:231.942383
LogP:8.32340
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN2672
Packing Group:III
Contact us for information like Aminodibenzanthrone chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Aminoviolanthrone physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Violanthrone,amino Use and application,EINECS 247-989-7 technical grade,usp/ep/jp grade.
Related News: INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment. Palladium(2+) nitrite ammoniate (1:2:2) manufacturer The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study. Palladium(2+) nitrite ammoniate (1:2:2) supplier In 2004, the National Development and Reform Commission, the former Ministry of Health, and the Ministry of Finance jointly formulated and promulgated the Administrative Measures for the Configuration and Use of Large Medical Equipment. This method uses X-ray electronic computer tomography (CT) devices and medical magnetic resonance imaging equipment, digital subtraction angiography X-ray machines of 800 mA or more as the management products of large-scale medical equipment, Purchase and use need to be reported to the provincial health administrative department for approval before approval. However, in recent years, with the comprehensive coverage of medical insurance, such basic medical facilities are being widely popularized, and the relevant implementation rules have directly linked the number of equipment to the number of doctors. Therefore, as the demand for imaging equipment continues to increase, it will have a greater boosting effect on the upstream contrast agent and its raw material market. Palladium(2+) nitrite ammoniate (1:2:2) vendor INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment. Palladium(2+) nitrite ammoniate (1:2:2) factory In 2004, the National Development and Reform Commission, the former Ministry of Health, and the Ministry of Finance jointly formulated and promulgated the Administrative Measures for the Configuration and Use of Large Medical Equipment. This method uses X-ray electronic computer tomography (CT) devices and medical magnetic resonance imaging equipment, digital subtraction angiography X-ray machines of 800 mA or more as the management products of large-scale medical equipment, Purchase and use need to be reported to the provincial health administrative department for approval before approval. However, in recent years, with the comprehensive coverage of medical insurance, such basic medical facilities are being widely popularized, and the relevant implementation rules have directly linked the number of equipment to the number of doctors. Therefore, as the demand for imaging equipment continues to increase, it will have a greater boosting effect on the upstream contrast agent and its raw material market.

