We serve Chemical Name:5-Bromonicotinamide CAS:28733-43-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
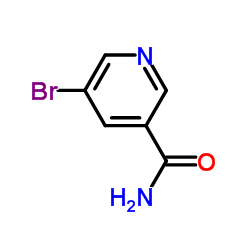
Chemical Name:5-Bromonicotinamide
CAS.NO:28733-43-9
Synonyms:5-Brompyridin-3-carboxamid;5-Bromonicotinamide;bromonicotinamide;BRT;5-Bromopyridine-3-carboxamide;3-Pyridinecarboxamide, 5-bromo-;Nicotinamide,5-bromo;3-bromonicotinamide;5-Bromo-nicotinamide;5-Bromo-3-pyridinecarboxamide;MFCD00173919;3-Bromo-5-carbamoylpyridine;EINECS 244-065-5;5-bromo-3-pyridine carboxamide
Molecular Formula:C6H5BrN2O
Molecular Weight:201.021
HS Code:2933399090
Physical and Chemical Properties:
Melting point:219-223 °C
Boiling point:315.5±27.0 °C at 760 mmHg
Density:1.7±0.1 g/cm3
Index of Refraction:1.614
PSA:55.98000
Exact Mass:199.958511
LogP:1.05
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like 5-Brompyridin-3-carboxamid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,5-bromo-3-pyridine carboxamide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-bromonicotinamide Use and application,MFCD00173919 technical grade,usp/ep/jp grade.
Related News: US citizens who have been to China in the last 14 days need to re-book to one of seven gateway airports: John F. Kennedy International Airport in New York, Los Angeles International Airport, Seattle, San Francisco International Airport, Chicago, Atlanta and Honolulu. 5-Bromonicotinamide manufacturer Drug manufacturers make medicines by mixing APIs and pharmaceutical excipients. 5-Bromonicotinamide supplier The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market. 5-Bromonicotinamide vendor An FDA inspection also turned up a long list of sanitary problems and bad manufacturing practices at the Emergent plant. 5-Bromonicotinamide factory The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market.

