We serve Chemical Name:Regadenoson CAS:313348-27-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
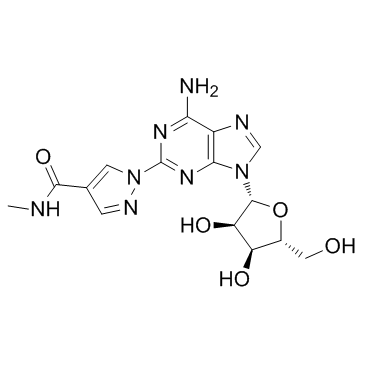
Chemical Name:Regadenoson
CAS.NO:313348-27-5
Synonyms:2-[4-(Methylcarbamoyl)-1H-pyrazol-1-yl]adenosine;Adenosine, 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]-;Regadenoson;Regadenoson anhydrous
Molecular Formula:C15H18N8O5
Molecular Weight:390.354
HS Code:
Physical and Chemical Properties:
Melting point:158-160ºC
Boiling point:N/A
Density:2.0±0.1 g/cm3
Index of Refraction:1.896
PSA:186.46000
Exact Mass:390.140015
LogP:-3.09
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-[4-(Methylcarbamoyl)-1H-pyrazol-1-yl]adenosine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Regadenoson anhydrous physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Regadenoson Use and application,Regadenoson technical grade,usp/ep/jp grade.
Related News: With the improvement of people’s living standards and the aging degree, the demand for medicines has been increasing for a long time. Therefore, the transfer from finished medicines to bulk medicines has greatly promoted the demand for bulk medicines. Regadenoson manufacturer No amount of unmet need can take the place of sufficient evidence, said Johns Hopkins public health professor Dr. Caleb Alexander, a member of the FDA advisory panel. Regadenoson supplier Onconova has conducted trials with two other research compounds and has a pre-clinical program with a CDK4/6 and Ark5 inhibitor, ON 123300. Regadenoson vendor The findings were published June 14 in the journal Nature Aging. Regadenoson factory With the improvement of people’s living standards and the aging degree, the demand for medicines has been increasing for a long time. Therefore, the transfer from finished medicines to bulk medicines has greatly promoted the demand for bulk medicines.

