We serve Chemical Name:1-(5-methoxypyridin-2-yl)ethanone CAS:325796-84-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
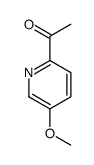
Chemical Name:1-(5-methoxypyridin-2-yl)ethanone
CAS.NO:325796-84-7
Synonyms:2-Acetyl-5-methoxypyridin;2-ACETYL-5-METHOXYPYRIDINE
Molecular Formula:C8H9NO2
Molecular Weight:151.16300
HS Code:2933399090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:39.19000
Exact Mass:151.06300
LogP:1.29280
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-Acetyl-5-methoxypyridin chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-ACETYL-5-METHOXYPYRIDINE physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Acetyl-5-methoxypyridin Use and application,2-ACETYL-5-METHOXYPYRIDINE technical grade,usp/ep/jp grade.
Related News: The primary endpoint of INSPIRE is overall survival. The trial continued beyond the pre-specified interim analysis and is nearing its conclusion. 1-(5-methoxypyridin-2-yl)ethanone manufacturer The pharmaceutical industry is related to the national economy and the people’s livelihood. The entire upstream and downstream areas involve all aspects of the national economy. Our government has a long-term direct or indirect policy preference for this. 1-(5-methoxypyridin-2-yl)ethanone supplier But the ban from Italy remains, Joseph Wu, Taiwan��s foreign minister, said on Sunday. 1-(5-methoxypyridin-2-yl)ethanone vendor The primary endpoint of INSPIRE is overall survival. The trial continued beyond the pre-specified interim analysis and is nearing its conclusion. 1-(5-methoxypyridin-2-yl)ethanone factory The primary endpoint of INSPIRE is overall survival. The trial continued beyond the pre-specified interim analysis and is nearing its conclusion.

