We serve Chemical Name:(S)-(+)-Tert-butylsulfinaMide CAS:343388-28-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
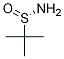
Chemical Name:(S)-(+)-Tert-butylsulfinaMide
CAS.NO:343388-28-3
Synonyms:(S)-(+)-Tert-butylsulfinaMide
Molecular Formula:C4H11NOS
Molecular Weight:121.20124
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:
Exact Mass:
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (S)-(+)-Tert-butylsulfinaMide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(S)-(+)-Tert-butylsulfinaMide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(S)-(+)-Tert-butylsulfinaMide Use and application,(S)-(+)-Tert-butylsulfinaMide technical grade,usp/ep/jp grade.
Related News: The Biogen Inc (BIIB.O) drug, Aduhelm, was authorized based on evidence that it can reduce brain plaques, a likely contributor to Alzheimer’s, rather than proof that it slows progression of the lethal mind-wasting disease. (S)-(+)-Tert-butylsulfinaMide manufacturer This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician��s Choice plus Best Supportive Care. (S)-(+)-Tert-butylsulfinaMide supplier Russia, which had temporarily stopped issuing work visas to Chinese citizens, is also halting visa-free entry for Chinese tour groups, the government said. Moscow has also stopped issuing electronic tourist visas to individual Chinese travelers. (S)-(+)-Tert-butylsulfinaMide vendor China considers self-ruled Taiwan to be part of its territory and has long sought to limit its diplomatic relations and recognition at international bodies such as the W.H.O. (S)-(+)-Tert-butylsulfinaMide factory This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician��s Choice plus Best Supportive Care.

