We serve Chemical Name:GDC-0349 CAS:1207360-89-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
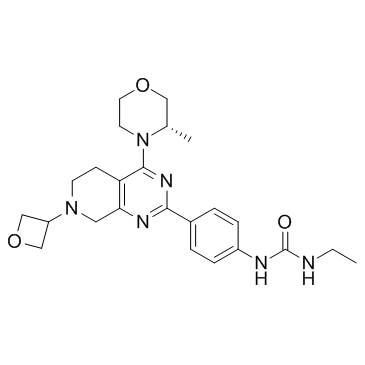
Chemical Name:GDC-0349
CAS.NO:1207360-89-1
Synonyms:(S)-1-ethyl-3-(4-(4-(3-methylmorpholino)-7-(oxetan-3-yl)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2-yl)phenyl)urea;Urea, N-ethyl-N’-[4-[5,6,7,8-tetrahydro-4-[(3S)-3-methyl-4-morpholinyl]-7-(3-oxetanyl)pyrido[3,4-d]pyrimidin-2-yl]phenyl]-;GDC-0349||GDC0349;1-Ethyl-3-(4-{4-[(3S)-3-methyl-4-morpholinyl]-7-(3-oxetanyl)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2-yl}phenyl)urea;GDC-0349
Molecular Formula:C24H32N6O3
Molecular Weight:452.549
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:571.3±50.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.620
PSA:95.34000
Exact Mass:452.253601
LogP:1.04
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (S)-1-ethyl-3-(4-(4-(3-methylmorpholino)-7-(oxetan-3-yl)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2-yl)phenyl)urea chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,GDC-0349 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Urea, N-ethyl-N’-[4-[5,6,7,8-tetrahydro-4-[(3S)-3-methyl-4-morpholinyl]-7-(3-oxetanyl)pyrido[3,4-d]pyrimidin-2-yl]phenyl]- Use and application,GDC-0349 technical grade,usp/ep/jp grade.
Related News: It has reserves in the direction of APIs and intermediates for lowering blood lipids, lowering blood sugar and anticoagulation. GDC-0349 manufacturer For both Black and white women, younger age and ER-negative breast cancer were risk factors in the genes most strongly associated with breast cancer, including BRCA1, BRCA2 and PALB2. GDC-0349 supplier But Sanofi’s rival CD38 antibody Sarclisa may soon be able to challenge Darzalex in the same front-line, transplant-ineligible population with the help of a more powerful backbone. GDC-0349 vendor It has reserves in the direction of APIs and intermediates for lowering blood lipids, lowering blood sugar and anticoagulation. GDC-0349 factory One key question for the fixed-duration combo is whether it can sustain remission after treatment has stopped. In the preliminary analysis of the GLOW trial, at three months after treatment ended, 51.9% of the Imbruvica-Venclexta patients had undetectable measurable residual disease (uMRD)—a stringent marker reflecting no signs of cancer—in bone marrow, versus 17.1% for the control group. The rates for peripheral blood were 54.7% and 39% for the two groups, respectively.

