We serve Chemical Name:quinolin-4-amine,hydrochloride CAS:35654-61-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
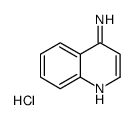
Chemical Name:quinolin-4-amine,hydrochloride
CAS.NO:35654-61-6
Synonyms:quinolin-4-amine hydrochloride
Molecular Formula:C9H9ClN2
Molecular Weight:180.63400
HS Code:2933499090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:39.64000
Exact Mass:180.04500
LogP:2.54910
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like quinolin-4-amine hydrochloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,quinolin-4-amine hydrochloride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,quinolin-4-amine hydrochloride Use and application,quinolin-4-amine hydrochloride technical grade,usp/ep/jp grade.
Related News: Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037. quinolin-4-amine,hydrochloride manufacturer According to statistics, in 2017, China’s total production of chemical drugs reached 3.478 million tons, a year-on-year increase of 1.6%. The main business income showed a steady increase, from 328.972 billion yuan in 2012 to 573.475 billion yuan in 2017. The total profit was 48.644 billion yuan, but profit margins remain low (8.48% in 2017). quinolin-4-amine,hydrochloride supplier In addition to APIs, a variety of pharmaceutical excipients are contained in the medicine. quinolin-4-amine,hydrochloride vendor Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037. quinolin-4-amine,hydrochloride factory Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.

