We serve Chemical Name:Fmoc-β-Ala-OH CAS:35737-10-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
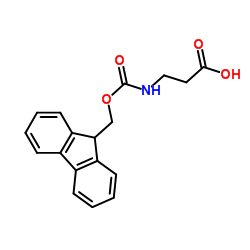
Chemical Name:Fmoc-β-Ala-OH
CAS.NO:35737-10-1
Synonyms:Fmoc–Ala-OH;N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-β-alanine;3-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}propanoic acid;Fmoc-β-Ala-OH;FMOC-b-Ala-OH;N-Fmoc-β-alanine;β-Alanine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-;Fmoc-b-Alanine;Fmoc-|A-alanine;MFCD00063328;3-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)propanoic acid;Fmoc-Beta-Ala-OH
Molecular Formula:C18H17NO4
Molecular Weight:311.332
HS Code:2924299090
Physical and Chemical Properties:
Melting point:142-147 °C
Boiling point:555.8±33.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.609
PSA:75.63000
Exact Mass:311.115753
LogP:3.36
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like Fmoc–Ala-OH chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Fmoc-Beta-Ala-OH physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,β-Alanine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]- Use and application,Fmoc-Beta-Ala-OH technical grade,usp/ep/jp grade.
Related News: The Ballina facility was first established in 1974 and was acquired by Charles River in 2002. Over the years it has added to its capabilities offering a comprehensive package of GMP services in support of recombinant biologics, vaccines, cell and gene therapies, biosimilars, and medical devices. Charles River now employs 230 people at two facilities in Ireland: the site in Ballina Co. Mayo which focuses on biologics testing, and a site in Dublin, established in 2017, which serves as the EMEA and APAC headquarters for the Company’s Microbial Solutions division. 1-ethyl-1-methylpyrrolidin-1-ium,hexafluorophosphate manufacturers AstraZeneca’s application for anifrolumab in SLE is under review by regulatory authorities in the US, EU and Japan, with decisions anticipated in the second half of 2021. Anifrolumab is not currently approved in any country. 1,1,3,3-Tetramethoxybutane suppliers as well as the antibody escape mutants for the current Emergency Use Authorization antibodies. Additionally, IGM-6268 was shown to be highly effective for prophylaxis and treatment in mouse models when administered intranasally. 4-methylsulfonyl-1,1-dioxothiolan-3-ol vendor & factory.

