We serve Chemical Name:(2RS)-2-(4-Ethylphenyl)propanoic acid CAS:3585-52-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
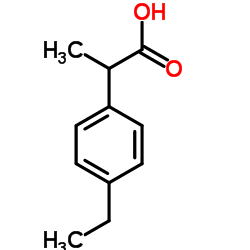
Chemical Name:(2RS)-2-(4-Ethylphenyl)propanoic acid
CAS.NO:3585-52-2
Synonyms:2-(4-Ethylphenyl)propanoic acid;UNII:7TM57OUE60;Benzeneacetic acid, 4-ethyl-α-methyl-;(2RS)-2-(4-Ethylphenyl)propanoic acid;Ibuprofen Impurity 14
Molecular Formula:C11H14O2
Molecular Weight:178.228
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:295.0±9.0 °C at 760 mmHg
Density:1.1±0.1 g/cm3
Index of Refraction:1.530
PSA:37.30000
Exact Mass:178.099380
LogP:2.84
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-(4-Ethylphenyl)propanoic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Ibuprofen Impurity 14 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(2RS)-2-(4-Ethylphenyl)propanoic acid Use and application,Ibuprofen Impurity 14 technical grade,usp/ep/jp grade.
Related News: Aduhelm was approved by the Food and Drug Administration on June 7. The drug, scientifically known as aducanumab, offers new hope to friends and families of patients living with the disease and is expected to generate billions of dollars in revenue for the company. benzimidoylamino-(4-chloro-benzimidoylamino)-methyl-sulfonium; bromide manufacturers This scheme has a total outlay of Rs. 6,940 crore for the entire period. The applications under four different target segments were invited on November 30, 2020. Of the 215 applications, 36 products across the 4 target segments were granted. N,N’-bis(3, 4-dimethoxybenzyl)-2,7-diaminofluorene suppliers Novartis and GSK carried out a swap of its vaccine and cancer drugs back in 2015. GSK paid $5.25 billion to Novartis for its vaccine business, and Novartis sent $16 billion for GSK’s oncology programs. Tafinlar and Mekinist had already been granted FDA approval at the time of that deal, and as a part of that deal, Novartis agreed to divest its own BRAF and MEK inhibitors to avoid creating a monopoly. rel-(5R,5aR,12aS,13S,15R,16aS)-9,15-dimethyldodecahydro-5H-5,13-methanoazonino[5,6-c][1,2,4]oxadiazolo[3,2-i]indol-2(1H)-one vendor & factory.

