We serve Chemical Name:3′-sialyllactose CAS:35890-38-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
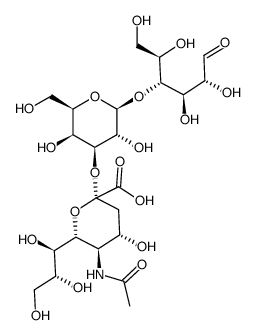
Chemical Name:3′-sialyllactose
CAS.NO:35890-38-1
Synonyms:N-acetylneuraminoyllactose;3′-Sialyllactose;3′-sialyllactose;3′-Sialyllactose (3′-SL);32-N-Acetyl-a-neuraminyllactose;3′-N-Acetylneuraminyl-D-lactose;3′-Monosialyllactose;3′-a-Sialyllactose;N-acetylneuraminosyl-D-lactose;3′-Sialyl-D-lactose;a2,3-Sialyllactose
Molecular Formula:C23H39NO19
Molecular Weight:633.55100
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:1132.8ºC at 760 mmHg
Density:1.72 g/cm3
Index of Refraction:
PSA:342.92000
Exact Mass:633.21200
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like N-acetylneuraminoyllactose chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,a2,3-Sialyllactose physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,a2,3-Sialyllactose Use and application,3′-Monosialyllactose technical grade,usp/ep/jp grade.
Related News: There’s a few factors that play into this. 3′-sialyllactose manufacturer In China, Wuhan used to be known as a city of cherry blossoms, an economic engine of the central heartland, and the birthplace of a century-old revolution that brought down the country’s last imperial dynasty. 3′-sialyllactose supplier ��Inceptua Medicines Access is delighted to be selected as Onconova��s partner for the Pre-approval Access Program for rigosertib. 3′-sialyllactose vendor The government will also send a charter flight with an Air New Zealand crew to repatriate up to 300 citizens in Wuhan. 3′-sialyllactose factory Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.

