We serve Chemical Name:diethyl tetrafluorosuccinate CAS:377-71-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
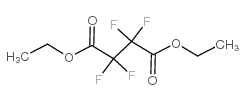
Chemical Name:diethyl tetrafluorosuccinate
CAS.NO:377-71-9
Synonyms:MFCD00015155;diethyl 2,2,3,3-tetrafluorobutanedioate;EINECS 206-821-2;Diethyl Tetrafluorosuccinate
Molecular Formula:C8H10F4O4
Molecular Weight:246.15600
HS Code:2917190090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:78°C 5mm
Density:1,273 g/cm3
Index of Refraction:1.368
PSA:52.60000
Exact Mass:246.05200
LogP:1.38320
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like MFCD00015155 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Diethyl Tetrafluorosuccinate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD00015155 Use and application,diethyl 2,2,3,3-tetrafluorobutanedioate technical grade,usp/ep/jp grade.
Related News: The Environmental Working Group, a nonprofit advocacy organization, applauded the bill’s introduction. diethyl tetrafluorosuccinate manufacturer As the first-to-market BTK inhibitor, Imbruvica has been treating front-line CLL patients since its monotherapy go-ahead in early 2016. But it’s now facing competition from AstraZeneca’s Calquence, which just detailed a head-to-head safety advantage over Imbruvica in previously treated CLL. diethyl tetrafluorosuccinate supplier The fine and functional chemicals sector provides new growth drivers. diethyl tetrafluorosuccinate vendor The fine and functional chemicals sector provides new growth drivers. diethyl tetrafluorosuccinate factory In 2016, in order to promote the innovative development, transformation and upgrading of the pharmaceutical industry, the Development and Reform Commission led the compilation of the Guiding Opinions on Promoting the Healthy Development of the Pharmaceutical Industry, which put forward requirements for all aspects of the medical industry and specifically proposed support for the field of chemical raw materials. .

