We serve Chemical Name:2-Phenoxytetrahydropyran CAS:4203-50-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
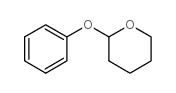
Chemical Name:2-Phenoxytetrahydropyran
CAS.NO:4203-50-3
Synonyms:2-phenoxyoxane;EINECS 224-114-7;2-PHENOXYTETRAHYDROPYRAN;Tetrahydro-2-phenoxy-2H-pyran
Molecular Formula:C11H14O2
Molecular Weight:178.22800
HS Code:2932999099
Physical and Chemical Properties:
Melting point:N/A
Boiling point:274.7ºC at 760 mmHg
Density:1.067 g/cm3
Index of Refraction:1.522
PSA:18.46000
Exact Mass:178.09900
LogP:2.59200
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-phenoxyoxane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Tetrahydro-2-phenoxy-2H-pyran physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-PHENOXYTETRAHYDROPYRAN Use and application,Tetrahydro-2-phenoxy-2H-pyran technical grade,usp/ep/jp grade.
Related News: In its message to the public, the FDA said that a person should consider being retested if they used Innova’s diagnostic within the past two weeks. 2-Phenoxytetrahydropyran manufacturer The iLet is designed to function as three medical devices in one. It can be configured as an insulin-only bionic pancreas, a glucagon-only bionic pancreas, or a bihormonal bionic pancreas using insulin and glucagon. 2-Phenoxytetrahydropyran supplier Inceptua Medicines Access is a business unit of the Inceptua Group. It offers full access solutions for the design, implementation and delivery of Pre-approval and Medicines Access Programs on behalf of biopharmaceutical companies. 2-Phenoxytetrahydropyran vendor The iLet is designed to function as three medical devices in one. It can be configured as an insulin-only bionic pancreas, a glucagon-only bionic pancreas, or a bihormonal bionic pancreas using insulin and glucagon. 2-Phenoxytetrahydropyran factory In its message to the public, the FDA said that a person should consider being retested if they used Innova’s diagnostic within the past two weeks.

