We serve Chemical Name:1-pyrimidin-2-ylimidazole-4-carbaldehyde CAS:433921-37-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
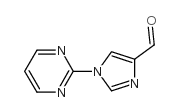
Chemical Name:1-pyrimidin-2-ylimidazole-4-carbaldehyde
CAS.NO:433921-37-0
Synonyms:or2129
Molecular Formula:C8H6N4O
Molecular Weight:174.15900
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:452.182ºC at 760 mmHg
Density:1.377g/cm3
Index of Refraction:1.689
PSA:60.67000
Exact Mass:174.05400
LogP:0.47480
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like or2129 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,or2129 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,or2129 Use and application,or2129 technical grade,usp/ep/jp grade.
Related News: The ability of a CDMO to lay down good quality audits, knowledge of equipment, packaging configurations, and good capability building of resources, helps in determining a competent CDMO partner. (+)-cephalosporolide E. manufacturers But when it came to deciding on whether Americans with compromised immune systems should get an extra dose of any COVID-19 vaccine, the CDC said it would need to wait for the FDA to give its regulatory thumbs up first. That could come either through amending its emergency use nods or awarding full approvals. 3-(5-chlorothiophenyl-2-sulfonyloxy)-5-methylphenol suppliers Novartis and GSK carried out a swap of its vaccine and cancer drugs back in 2015. GSK paid $5.25 billion to Novartis for its vaccine business, and Novartis sent $16 billion for GSK’s oncology programs. Tafinlar and Mekinist had already been granted FDA approval at the time of that deal, and as a part of that deal, Novartis agreed to divest its own BRAF and MEK inhibitors to avoid creating a monopoly. 3-(diethylamino)-4-methyl-5-(phenylthio)benzo[f][1,2]thiazepine 1,1-dioxide vendor & factory “We welcome a formal review into the interactions between the FDA and Biogen on the path to the approval of aducanumab,” Sandrock said. “A better understanding of the facts is good for everyone involved to assure confidence in both the therapy and the process by which it was approved as we prioritize the issues that affect patients.”,Novartis and GSK carried out a swap of its vaccine and cancer drugs back in 2015. GSK paid $5.25 billion to Novartis for its vaccine business, and Novartis sent $16 billion for GSK’s oncology programs. Tafinlar and Mekinist had already been granted FDA approval at the time of that deal, and as a part of that deal, Novartis agreed to divest its own BRAF and MEK inhibitors to avoid creating a monopoly.

