We serve Chemical Name:Octadecylbenzene CAS:4445-07-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
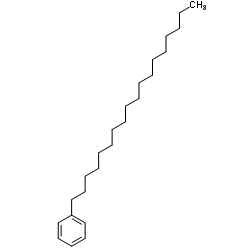
Chemical Name:Octadecylbenzene
CAS.NO:4445-07-2
Synonyms:Octadecane, 1-phenyl-;1-Phenyloctadecane;Stearylbenzene;n-Octadecylbenzene;Benzene, octadecyl-;Octadecylbenzene;MFCD00048500;EINECS 224-684-7
Molecular Formula:C24H42
Molecular Weight:330.590
HS Code:2902909090
Physical and Chemical Properties:
Melting point:32-36 °C
Boiling point:408.2±8.0 °C at 760 mmHg
Density:0.9±0.1 g/cm3
Index of Refraction:1.481
PSA:
Exact Mass:330.328644
LogP:11.71
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like Octadecane, 1-phenyl- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,EINECS 224-684-7 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Stearylbenzene Use and application,1-Phenyloctadecane technical grade,usp/ep/jp grade.
Related News: The company’s product reserves are rich, with more than one hundred varieties in various categories from intermediates to APIs. At present, the grays and anti-hepatitis C series intermediates are steadily advancing, which will ensure future growth. Octadecylbenzene manufacturer Government prosecutors often consider whether a corporation made a genuine effort to investigate and address potentially illegal activities once learning of them. Octadecylbenzene supplier The Branchburg factory first came under FDA scrutiny in late 2019, when agency inspectors began to document numerous quality control problems. By March, 2020, the FDA had deemed the manufacturing issues as “Official Action Indicated,” its most serious category of violation. Octadecylbenzene vendor The Branchburg factory first came under FDA scrutiny in late 2019, when agency inspectors began to document numerous quality control problems. By March, 2020, the FDA had deemed the manufacturing issues as “Official Action Indicated,” its most serious category of violation. Octadecylbenzene factory The U.S. Food & Drug Administration on Friday said Johnson & Johnson (JNJ.N) must throw away millions of doses of its COVID-19 vaccine that were manufactured at a problem-plagued Baltimore factory but also cleared millions for use.

