We serve Chemical Name:Butane-1,4-diyldiphosphonic acid CAS:4671-77-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
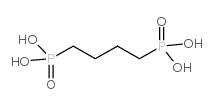
Chemical Name:Butane-1,4-diyldiphosphonic acid
CAS.NO:4671-77-6
Synonyms:1,2-butylenediphosphonic acid;1,4-Diphosphonobutane;1,4-butylene-diphosphonic acid;Butane-1,4-diphosphonic acid;1,4-Butanediphosphonic acid;MFCD01631212;butane-1,4-diyldiphosphonic acid;1,4-butylenediphosphonic avid
Molecular Formula:C4H12O6P2
Molecular Weight:218.08200
HS Code:
Physical and Chemical Properties:
Melting point:219-221ºC
Boiling point:544.4ºC at 760 mmHg
Density:N/A
Index of Refraction:
PSA:134.68000
Exact Mass:218.01100
LogP:0.12200
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1,2-butylenediphosphonic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1,4-butylenediphosphonic avid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,4-butylene-diphosphonic acid Use and application,1,4-Butanediphosphonic acid technical grade,usp/ep/jp grade.
Related News: Zynteglo is a one-time treatment for a blood disorder known as beta thalassaemia in patients 12 years and older who require regular blood transfusions. Benzoic acid, 2-(1,3-benzodioxol-5-yloxy)-6-(4-methylphenoxy)- manufacturers Alexion has been working to push Ultomiris through additional indications ahead of Soliris’ expected loss of exclusivity in 2025. N-butan-2-yl-1-cyclohexyl-5-oxopyrrolidine-3-carboxamide suppliers This will entail workforce hiring of over 110 personnel too. N4-cyclohexyl-pyrimidine-4,6-diyldiamine vendor & factory Zynteglo is a one-time treatment for a blood disorder known as beta thalassaemia in patients 12 years and older who require regular blood transfusions.,Alexion has been working to push Ultomiris through additional indications ahead of Soliris’ expected loss of exclusivity in 2025.

