We serve Chemical Name:Cinobufagin CAS:470-37-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
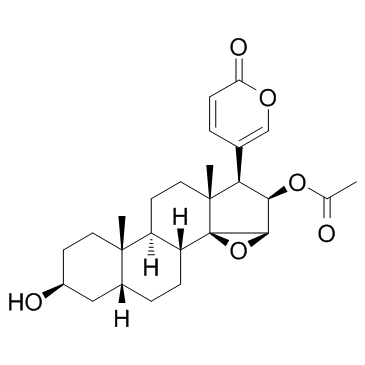
Chemical Name:Cinobufagin
CAS.NO:470-37-1
Synonyms:Bufa-20,22-dienolide, 16-(acetyloxy)-14,15-epoxy-3-hydroxy-, (3β,5β,15β,16β)-;(3β,5β,15β,16β)-16-Acetoxy-3-hydroxy-14,15-epoxybufa-20,22-dienolide;5β-Bufa-20,22-dienolide, 14,15β-epoxy-3β,16β-dihydroxy-, 16-acetate (8CI);cinobufagine;Bufa-20,22-dienolide, 16- (acetyloxy)-14,15-epoxy-3-hydroxy-, (3β,5β,15β,16β)-;CINOBUFAGIN(P);UNII:T9PSN4R8IR;5β-Bufa-20,22-dienolide, 14,15β-epoxy-3β,16β-dihydroxy-, 16-acetate;MFCD00056825;RARECHEM BK HC T302;Cinobufagin;(3β,5β,15β,16β)-16-(acetyloxy)-3-hydroxy-14,15-epoxybufa-20,22-dienolide
Molecular Formula:C26H34O6
Molecular Weight:442.545
HS Code:3001200090
Physical and Chemical Properties:
Melting point:222-223ºC
Boiling point:595.4±50.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.595
PSA:89.27000
Exact Mass:442.235535
LogP:2.43
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2811
Packing Group:II
Contact us for information like Bufa-20,22-dienolide, 16-(acetyloxy)-14,15-epoxy-3-hydroxy-, (3β,5β,15β,16β)- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(3β,5β,15β,16β)-16-(acetyloxy)-3-hydroxy-14,15-epoxybufa-20,22-dienolide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD00056825 Use and application,Bufa-20,22-dienolide, 16-(acetyloxy)-14,15-epoxy-3-hydroxy-, (3β,5β,15β,16β)- technical grade,usp/ep/jp grade.
Related News: Taking Minuo Huawei as an example, in 2016, the capacity utilization rate and production-sales ratio of its multiple drug substance varieties reached more than 90%. Therefore, domestic API companies have expanded their production capacity in recent years to meet the growing demand for APIs. Cinobufagin manufacturer The FDA said its decision allows for the J&J doses to be used in the United States or exported. T Cinobufagin supplier The FDA said its decision allows for the J&J doses to be used in the United States or exported. T Cinobufagin vendor The FDA said its decision allows for the J&J doses to be used in the United States or exported. T Cinobufagin factory Many branded versions of drugs are currently more expensive in China than in other major markets. They could now be subjected to a centralized procurement program where manufacturers will have to go through a bidding process to get the right to supply drugs to public hospitals, the National Health Commission said in a document published on late Friday.

