We serve Chemical Name:Simvastatin Dimer Impurity CAS:476305-24-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
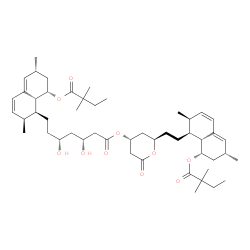
Chemical Name:Simvastatin Dimer Impurity
CAS.NO:476305-24-5
Synonyms:Butanoic acid, 2,2-dimethyl-, (1R,3S,7R,8R,8aS)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4S)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester;(1R,3S,7R,8R,8aS)-8-{2-[(2R,4S)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-naphthalenyl 2,2-dimethylbutanoate
Molecular Formula:C50H76O10
Molecular Weight:837.132
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:882.067°C at 760 mmHg
Density:1.141g/cm3
Index of Refraction:1.547
PSA:72.83000
Exact Mass:836.543823
LogP:4.41
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Butanoic acid, 2,2-dimethyl-, (1R,3S,7R,8R,8aS)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4S)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(1R,3S,7R,8R,8aS)-8-{2-[(2R,4S)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-naphthalenyl 2,2-dimethylbutanoate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(1R,3S,7R,8R,8aS)-8-{2-[(2R,4S)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-naphthalenyl 2,2-dimethylbutanoate Use and application,Butanoic acid, 2,2-dimethyl-, (1R,3S,7R,8R,8aS)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4S)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester technical grade,usp/ep/jp grade.
Related News: Genzyme Pharmaceuticals develops and manufactures chemically synthesized pharmaceutical materials and technologies for the global pharmaceutical industry and focuses on lipids, peptides, carbohydrates, oligonucleotides, and custom small molecules. Simvastatin Dimer Impurity manufacturer The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market. Simvastatin Dimer Impurity supplier INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment. Simvastatin Dimer Impurity vendor The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market. Simvastatin Dimer Impurity factory The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market.

