We serve Chemical Name:1,8,9-TRIHYDROXYANTHRACENE CAS:480-22-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
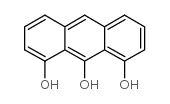
Chemical Name:1,8,9-TRIHYDROXYANTHRACENE
CAS.NO:480-22-8
Synonyms:1,8,9-Trihydroxyanthracene;MFCD00001250
Molecular Formula:C14H10O3
Molecular Weight:226.22700
HS Code:2907299090
Physical and Chemical Properties:
Melting point:181 °C
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:60.69000
Exact Mass:226.06300
LogP:3.10980
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1,8,9-Trihydroxyanthracene chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD00001250 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,8,9-Trihydroxyanthracene Use and application,MFCD00001250 technical grade,usp/ep/jp grade.
Related News: The chemical compound which is in the process of becoming an API from a raw material is called an intermediate. 1,8,9-TRIHYDROXYANTHRACENE manufacturer Delta moved the date up after the US State Department warned that people should not travel to China due to concerns about the spread of coronavirus, which was first discovered in Wuhan, Hubei province, in December. 1,8,9-TRIHYDROXYANTHRACENE supplier The chemical compound which is in the process of becoming an API from a raw material is called an intermediate. 1,8,9-TRIHYDROXYANTHRACENE vendor The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy. 1,8,9-TRIHYDROXYANTHRACENE factory The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.

