We serve Chemical Name:DL-LAUDANOSOLINE HYDROBROMIDE TRIHYDRATE CAS:485-33-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
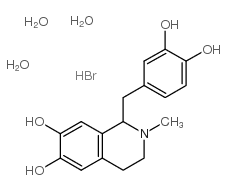
Chemical Name:DL-LAUDANOSOLINE HYDROBROMIDE TRIHYDRATE
CAS.NO:485-33-6
Synonyms:D,L-laudanosoline;EINECS 207-613-4;(R,S)-laudanosoline;(R,S)-laundanosoline;DL-LaudanosolineHBr;(+-)-1-(3,4-dihydroxy-benzyl)-2-methyl-1,2,3,4-tetrahydro-isoquinoline-6,7-diol;MFCD00167305;laudanosoline;LAUDANOSOLINE HYDROBROMIDE TRIHYDRATE;1-[(3,4-Dihydroxyphenyl)methyl]-1,2,3,4-tetrahydro-2-methyl-6,7-isoquinolinediol;DL-LAUDANOSOLINE HYDROBROMIDE;DL-6,7-dimethoxy-1-((3,4-dimethoxyphenyl)methyl)-2-methylisoquinoline hydrobromide trihydrate;DL-laudanosine hydrobromide trihydrate
Molecular Formula:C17H26BrNO7
Molecular Weight:436.29500
HS Code:
Physical and Chemical Properties:
Melting point:232-234ºC(lit.)
Boiling point:548ºC at 760mmHg
Density:1.373g/cm3
Index of Refraction:
PSA:111.85000
Exact Mass:435.08900
LogP:2.98380
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2811 6.1/PG 3
Packing Group:
Contact us for information like D,L-laudanosoline chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,DL-laudanosine hydrobromide trihydrate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1-[(3,4-Dihydroxyphenyl)methyl]-1,2,3,4-tetrahydro-2-methyl-6,7-isoquinolinediol Use and application,(R,S)-laundanosoline technical grade,usp/ep/jp grade.
Related News: In addition, the FDA approval ignored the recommendation of its outside advisors, who said Biogen did not provide enough evidence of clinical benefit. Three of the advisory panel’s members have resigned in protest since the FDA decision was announced on Monday. DL-LAUDANOSOLINE HYDROBROMIDE TRIHYDRATE manufacturer The memo denies the company made false statements to the FDA. In their complaint, employees said they were broadly concerned that quality control documents the FDA requires companies to maintain had been rewritten or fabricated. The employees did not specify whether these materials had been shown to the FDA. DL-LAUDANOSOLINE HYDROBROMIDE TRIHYDRATE supplier Inceptua Medicines Access is a business unit of the Inceptua Group. It offers full access solutions for the design, implementation and delivery of Pre-approval and Medicines Access Programs on behalf of biopharmaceutical companies. DL-LAUDANOSOLINE HYDROBROMIDE TRIHYDRATE vendor In addition, the FDA approval ignored the recommendation of its outside advisors, who said Biogen did not provide enough evidence of clinical benefit. Three of the advisory panel’s members have resigned in protest since the FDA decision was announced on Monday. DL-LAUDANOSOLINE HYDROBROMIDE TRIHYDRATE factory The memo denies the company made false statements to the FDA. In their complaint, employees said they were broadly concerned that quality control documents the FDA requires companies to maintain had been rewritten or fabricated. The employees did not specify whether these materials had been shown to the FDA.

