We serve Chemical Name:(2-Iodophenyl)hydrazine CAS:50914-15-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
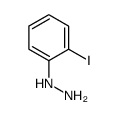
Chemical Name:(2-Iodophenyl)hydrazine
CAS.NO:50914-15-3
Synonyms:o-IC6H4N2H3;2-iodophenylhydrazine;(2-Jod-phenyl)-hydrazin
Molecular Formula:C6H7IN2
Molecular Weight:234.03800
HS Code:2928000090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:38.05000
Exact Mass:233.96500
LogP:2.35010
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like o-IC6H4N2H3 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(2-Jod-phenyl)-hydrazin physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(2-Jod-phenyl)-hydrazin Use and application,2-iodophenylhydrazine technical grade,usp/ep/jp grade.
Related News: CHMP on Friday also announced that Roche pulled its Tecentriq application in triple-negative breast cancer after it noted that the EMA said the results from a late-stage trial do not favor the drug’s benefit-risk calculation in this indication. 3-{4-[3-(4-nitro-phenyl)-propyl]-piperazin-1-yl}-1,2-benzisothiazol manufacturers In 2021, the combo has raked in $818 million in the first half of the year alone for Novartis, a number that is already up 11% from last year. Comparatively, Roche reported $218 million in sales of Zelboraf in 2016, and revenue was not reported in its most recent mid-year update. p-(2-Chlor-aethoxy)-acetophenon-(2.4-dinitro-phenylhydrazon) suppliers CHMP on Friday also announced that Roche pulled its Tecentriq application in triple-negative breast cancer after it noted that the EMA said the results from a late-stage trial do not favor the drug’s benefit-risk calculation in this indication. 6-amino-9-(O3,O5-diacetyl-β-D-arabinofuranosyl)-7,9-dihydro-purine-8-thione vendor & factory CHMP on Friday also announced that Roche pulled its Tecentriq application in triple-negative breast cancer after it noted that the EMA said the results from a late-stage trial do not favor the drug’s benefit-risk calculation in this indication. ,For its part, the FDA’s representative at the meeting, Doran Fink, M.D., said the drug regulator is “working as rapidly as possible to conduct a thorough and comprehensive” review of all the COVID-19 vaccines that have applied for full approval. Pfizer and Moderna recently submitted their applications for a full nod.

