We serve Chemical Name:m-PEG5-SH CAS:524030-00-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
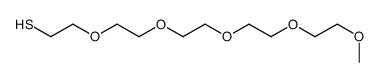
Chemical Name:m-PEG5-SH
CAS.NO:524030-00-0
Synonyms:3,6,9,12,15-pentaoxahexadecane-1-thiol
Molecular Formula:C11H24O5S
Molecular Weight:268.37000
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:1.048
Index of Refraction:
PSA:84.95000
Exact Mass:268.13400
LogP:0.62900
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 3,6,9,12,15-pentaoxahexadecane-1-thiol chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3,6,9,12,15-pentaoxahexadecane-1-thiol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3,6,9,12,15-pentaoxahexadecane-1-thiol Use and application,3,6,9,12,15-pentaoxahexadecane-1-thiol technical grade,usp/ep/jp grade.
Related News: Resonant exploits its proprietary IMPaCT tumor microenvironment models and data platform to discover novel, unappreciated targets and functionally active anti-tumor antibodies for difficult to treat tumors. m-PEG5-SH manufacturer The complete chemical and pharmaceutical industry chain consists of basic chemical raw materials, pharmaceutical intermediates, chemical raw materials and chemical preparations. m-PEG5-SH supplier Resonant exploits its proprietary IMPaCT tumor microenvironment models and data platform to discover novel, unappreciated targets and functionally active anti-tumor antibodies for difficult to treat tumors. m-PEG5-SH vendor China has a complete upstream and downstream industry chain, lower costs, and good talent reserves. It is one of the few industries in China that has an advantage in international competition. m-PEG5-SH factory The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented.

