We serve Chemical Name:Mesity aceti acid CAS:52629-46-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
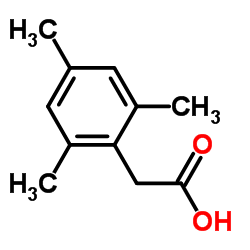
Chemical Name:Mesity aceti acid
CAS.NO:52629-46-6
Synonyms:Benzeneacetic acid, 2,4,6-trimethyl-;Mesitylacetic acid;Mesity aceti acid
Molecular Formula:C11H14O2
Molecular Weight:178.228
HS Code:2915900090
Physical and Chemical Properties:
Melting point:167-168ºC
Boiling point:312.9±11.0 °C at 760 mmHg
Density:1.1±0.1 g/cm3
Index of Refraction:1.538
PSA:37.30000
Exact Mass:178.099380
LogP:2.88
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Benzeneacetic acid, 2,4,6-trimethyl- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Mesity aceti acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Benzeneacetic acid, 2,4,6-trimethyl- Use and application,Mesitylacetic acid technical grade,usp/ep/jp grade.
Related News: Johnson & Johnson’s COVID-19 vaccine presents greater benefits than it does safety risks, especially amid the quickly spreading Delta variant, a key CDC expert panel decided. However, the panel said that a ruling over the need for a booster added to all COVID shots will have to start with the FDA. 6-((4-cyano-2-fluorophenyl)amino)-N-(cyclobutylmethyl)-4-isopropylnicotinamide manufacturers The CDC’s Advisory Committee on Immunization Practices (ACIP) decision on Thursday came after an hours-long discussion over a handful of Guillain-Barré syndrome (GBS) cases reported after J&J’s jab. The independent group of experts were also tasked with reviewing the need for booster shots, specifically for people with compromised immune systems. Retropepsin (human immunodeficiency virus 1 strain 29 gene polfragment) suppliers Meanwhile, EMA’s Committee for Medicinal Products for Human Use adopted a positive opinion recommending marketing authorization for Moderna’s Covid-19 vaccine, now known as Spikevax, to include adolescents 12 years of age and older. (6-hydroperoxy-1-hydroxy-14,24,44,53,64,73,83,94-pentaoxolo[2,1]hexaoxin-2-yl)(13,34,43,53-pentaoxol-3-yl)-4-oxidanone vendor & factory.

