We serve Chemical Name:2,2′,3,3′,4,5′-Hexachlorobiphenyl CAS:52663-66-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
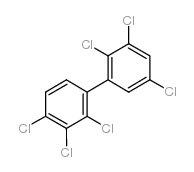
Chemical Name:2,2′,3,3′,4,5′-Hexachlorobiphenyl
CAS.NO:52663-66-8
Synonyms:1,2,3-trichloro-4-(2,3,5-trichlorophenyl)benzene
Molecular Formula:C12H4Cl6
Molecular Weight:360.87800
HS Code:2903999090
Physical and Chemical Properties:
Melting point:115.76°C (estimate)
Boiling point:402.9ºC at 760 mmHg
Density:1.593 g/cm3
Index of Refraction:1.626
PSA:
Exact Mass:357.84400
LogP:7.27400
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1,2,3-trichloro-4-(2,3,5-trichlorophenyl)benzene chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1,2,3-trichloro-4-(2,3,5-trichlorophenyl)benzene physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,2,3-trichloro-4-(2,3,5-trichlorophenyl)benzene Use and application,1,2,3-trichloro-4-(2,3,5-trichlorophenyl)benzene technical grade,usp/ep/jp grade.
Related News: A thorough safety and risk analysis procedure should be administered at an appropriate time with documentation that adheres to the rules and regulations set forth by the regulatory authorities. N-methoxy-N-methyl-4-morpholin-4-ylbenzamide manufacturers A multitude of enzymes is now utilized for generating active pharmaceutical ingredients (APIs) and intermediates for their production, with such reagents offering numerous advantages compared to using traditional metal catalysts. 4-naphthalen-1-yliminobutan-2-ol suppliers SARS-CoV-2 has evolved mutations that severely compromise the neutralizing activities of multiple IgG monoclonal antibodies, including those under clinical trials and authorized for emergency use.
Therefore, developing new antibody therapies that can overcome these challenges is an urgent unmet need, and we are pleased with the data published today,” An said.
“Synergizing the strengths of multiple institutions from academia and industry is the key to the rapid translation from ideas to therapeutic candidates.
This is another example of such success. The cross-institutional and academic-industry collaborations should be expanded to other disease indications,” said Pei-Yong Shi, PhD, professor and co-senior author of the study from the Department of Biochemistry and Molecular Biology at UTMB Health.
This antibody has been licensed to biotech partner IGM Biosciences for drug development.
“The ability to use potently neutralizing IgM antibodies against SARS-CoV-2 with broad coverage of VOCs, VOIs, and viral escape mutants, is a very exciting application of the IGM platform,” said Fred Schwarzer, CEO of IGM Biosciences.
“We are grateful to our collaborators at UTHealth, UTMB Health, and our scientists at IGM for the exceptional work described in Nature today. N,N-dimethylmethanamine,methoxycarbonylboron vendor & factory.
Therefore, developing new antibody therapies that can overcome these challenges is an urgent unmet need, and we are pleased with the data published today,” An said.
“Synergizing the strengths of multiple institutions from academia and industry is the key to the rapid translation from ideas to therapeutic candidates.
This is another example of such success. The cross-institutional and academic-industry collaborations should be expanded to other disease indications,” said Pei-Yong Shi, PhD, professor and co-senior author of the study from the Department of Biochemistry and Molecular Biology at UTMB Health.
This antibody has been licensed to biotech partner IGM Biosciences for drug development.
“The ability to use potently neutralizing IgM antibodies against SARS-CoV-2 with broad coverage of VOCs, VOIs, and viral escape mutants, is a very exciting application of the IGM platform,” said Fred Schwarzer, CEO of IGM Biosciences.
“We are grateful to our collaborators at UTHealth, UTMB Health, and our scientists at IGM for the exceptional work described in Nature today. N,N-dimethylmethanamine,methoxycarbonylboron vendor & factory.

