We serve Chemical Name:ent-NADPH CAS:53-57-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
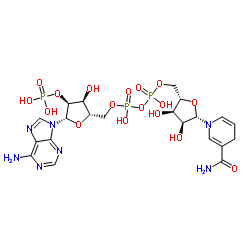
Chemical Name:ent-NADPH
CAS.NO:53-57-6
Synonyms:ent-NADPH;[[(2S,3S,4S,5S)-5-(6-aminopurin-9-yl)-3-hydroxy-4-phosphonooxy-te trahydrofuran-2-yl]methoxy-hydroxy-phosphoryl] [(2S,3R,4S,5S)-5-( 3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl]m ethyl hydrogen phosphate;Di-t-butyl iminodicarboxylate;tert-Butyl iminodicarboxylate;N-Boc-tert-butylcarbamate;di-tert-butyl imidodicarbonate;Iminodicarboxylic Acid Di-tert-butyl Ester;Di-tert-butyl-iminodicarboxylate;di-tert-butyl iminodicarbonate
Molecular Formula:C21H30N7O17P3
Molecular Weight:745.421
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:1175.1±75.0 °C at 760 mmHg
Density:2.3±0.1 g/cm3
Index of Refraction:1.849
PSA:394.57000
Exact Mass:745.091125
LogP:-5.93
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like ent-NADPH chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,di-tert-butyl iminodicarbonate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,ent-NADPH Use and application,tert-Butyl iminodicarboxylate technical grade,usp/ep/jp grade.
Related News: Our pharma and biotech offering includes registration and commercialization of products through in-licensing and flexible partnerships. ent-NADPH manufacturer Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). A novel coronavirus (nCoV) is a new strain that has not been previously identified in humans. ent-NADPH supplier In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials. ent-NADPH vendor The Company has established a leadership position in the clinical development and manufacture of universal, off-the-shelf cell products using its proprietary induced pluripotent stem cell (iPSC) product platform. ent-NADPH factory Our pharma and biotech offering includes registration and commercialization of products through in-licensing and flexible partnerships.

