We serve Chemical Name:(2-Amino-5-iodophenyl)methanol CAS:53279-83-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
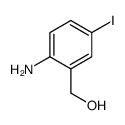
Chemical Name:(2-Amino-5-iodophenyl)methanol
CAS.NO:53279-83-7
Synonyms:2-amino-5-iodobenzyl alcohol;2-amino-5-fluorophenyl 4-pyridyl ketone
Molecular Formula:C7H8INO
Molecular Weight:249.04900
HS Code:2922199090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:46.25000
Exact Mass:248.96500
LogP:1.94690
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-amino-5-iodobenzyl alcohol chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-amino-5-fluorophenyl 4-pyridyl ketone physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-amino-5-fluorophenyl 4-pyridyl ketone Use and application,2-amino-5-fluorophenyl 4-pyridyl ketone technical grade,usp/ep/jp grade.
Related News: Around 57% of Israel’s 9.3 million population has been vaccinated. Around 160 people are hospitalised with severe symptoms and daily new infections have spiked to more than 2,000, up from a handful of cases per day a few months ago. 2,4-dichloro-5-phenylphenol manufacturers It’s official. Nearly two years after the FDA granted conditional approval to Merck’s Keytruda and Eisai’s Lenvima for endometrial cancer, the U.S. regulator has blessed the combination therapy, with no strings attached. 4,4-Dichloro-2,6-dimethoxy-3,5-diphenyl-4λ4-[1,4]oxaselenane suppliers TULIP-2 assessed the effect of anifrolumab in reducing disease activity as measured by the BILAG-Based Composite Lupus Assessment (BICLA) scale. In TULIP-1, 457 eligible patients were randomised (1:2:2) and received a fixed-dose intravenous infusion of 150mg anifrolumab, 300mg anifrolumab or placebo every four weeks, 1-(2-(tert-butyl)-6-(trifluoromethyl)pyrimidin-4-yl)-4-(2-(((4-methyl-5-(pyrazin-2-yl)-4H-1,2,4-triazol-3-yl)thio)methyl)allyl)-1,4-diazepane hydrochloride vendor & factory.

