We serve Chemical Name:8-azidoadenosine 5′-triphosphate, sodium salt CAS:53696-59-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
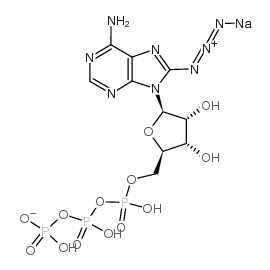
Chemical Name:8-azidoadenosine 5′-triphosphate, sodium salt
CAS.NO:53696-59-6
Synonyms:Adenosine 5′-(tetrahydrogen triphosphate),8-azido;8-Azido-D-adenosine5′-triphosphatesodiumsalt;8-AZIDOADENOSINE-5′-O-TRIPHOSPHATE SODIUM SALT;8-N3-ATP SODIUM SALT
Molecular Formula:C10H14N8NaO13P3
Molecular Weight:570.17500
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:336.11000
Exact Mass:569.97900
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Adenosine 5′-(tetrahydrogen triphosphate),8-azido chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,8-N3-ATP SODIUM SALT physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,8-N3-ATP SODIUM SALT Use and application,8-N3-ATP SODIUM SALT technical grade,usp/ep/jp grade.
Related News: Only when the drug substance is processed into a pharmaceutical preparation can it become a drug for clinical application. 8-azidoadenosine 5′-triphosphate, sodium salt manufacturer Like other countries in Southeast Asia, Indonesia depends heavily on Chinese tourism. On Thursday alone, 10,000 Chinese tourists canceled their trips to Bali, according to one industry association. 8-azidoadenosine 5′-triphosphate, sodium salt supplier Only when the drug substance is processed into a pharmaceutical preparation can it become a drug for clinical application. 8-azidoadenosine 5′-triphosphate, sodium salt vendor The new restrictions on foreign nationals begin on February 3, Prime Minister Jacinda Ardern announced in a press release Sunday. 8-azidoadenosine 5′-triphosphate, sodium salt factory Only when the drug substance is processed into a pharmaceutical preparation can it become a drug for clinical application.

